Allegheny College
Abstract
Sgs1p in Saccharomyces cerevisiae belongs to the RecQ helicase family. Sgs1p is involved in recombination during DNA damage repair and sumoylation of Sgs1p is one mechanism by which the protein is regulated. To further understand the significance of Sgs1p sumoylation in DNA damage repair, we examined the genetic interaction between SGS1 SUMO mutants and a mutant of SRS2, the protein product of which also prevents aberrant recombination structures. We observed that SGS1-SuOff, a mutant in which Sgs1p cannot be sumoylated, attenuates the mild sensitivity of srs2Δ cells to methyl methane sulfonate.
Description
The RecQ helicases play a crucial role in the maintenance of genome stability. The importance of these helicases is most apparent in genetic diseases associated with RecQ helicases. In human, defects in BLM, RECQL4 or WRN result in Bloom’s syndrome, Rothmund–Thomson syndrome and Werner’s syndrome, respectively (Ellis et al. 1995; Yu et al. 1996; Kitao et al. 1999). A common characteristic of all these syndromes is a predisposition to genome stability and cancer (Croteau et al. 2014). In Saccharomyces cerevisiae, Sgs1p is a RecQ helicase homolog. Sgs1p functions as part of the STR complex (Sgs1p-Top3p-Rmi1p complex) and regulates recombination to prevent illegitimate structures (Croteau et al. 2014). For instance, cells deficient in SGS1 accumulated Rad51p-dependent aberrant DNA structures at replication forks when cells were treated with methylmethane sulfonate (MMS) (Liberi et al. 2005). How Sgs1p regulates recombination, in part, depends on sumoylation, a process in which a SUMO (small ubiquitin-like modifier) peptide is covalently conjugated to target proteins. In response to DNA damage, Sgs1p becomes sumoylated (Branzei et al. 2006; Bermúdez-López et al. 2016; Bonner et al.. 2016). Sumoylation of Sgs1p is mediated by an E3 SUMO ligase, Mms21p and this modification supports the ability of Sgs1p to resolve aberrant DNA structures at replication forks (Branzei et al. 2006; Bermúdez-López et al. 2016; Bonner et al. 2016).
Although recombination events help to overcome the impediments at replication forks, failure to regulate these processes can become the source of genome instability. Thus, it is not surprising that more than one pathway function at replication forks to prevent illegitimate recombination. Srs2p is another helicase responsible for inhibiting aberrant DNA structures in S. cerevisiae. Although Sgs1p and Srs2p are both 3’-to-5’ helicases and generally considered to be anti-recombinogenic, almost synthetic lethal phenotype of sgs1 srs2 mutants implies that they function in independent pathways (Gangloff et al. 2000; Marini and Krejci 2010). Consistent with this idea, a recent study detailing the homologous recombination (HR) process involving Sgs1p and Srs2p uncovered that these helicases target different types of D-loops during HR (Piazza et al. 2019). However, genetic data suggest that Sgs1p and Srs2p have some overlapping functions. Sgs1p can suppress Srs2p deficiency in a dose-dependent manner suggesting that Sgs1p may partially compensate for the loss of Srs2p (Mankouri et al. 2002). Likewise, overexpression of Srs2p in sgs1Δ reduces MMS-induced accumulation of X-molecules at replication forks (Liberi et al. 2005).
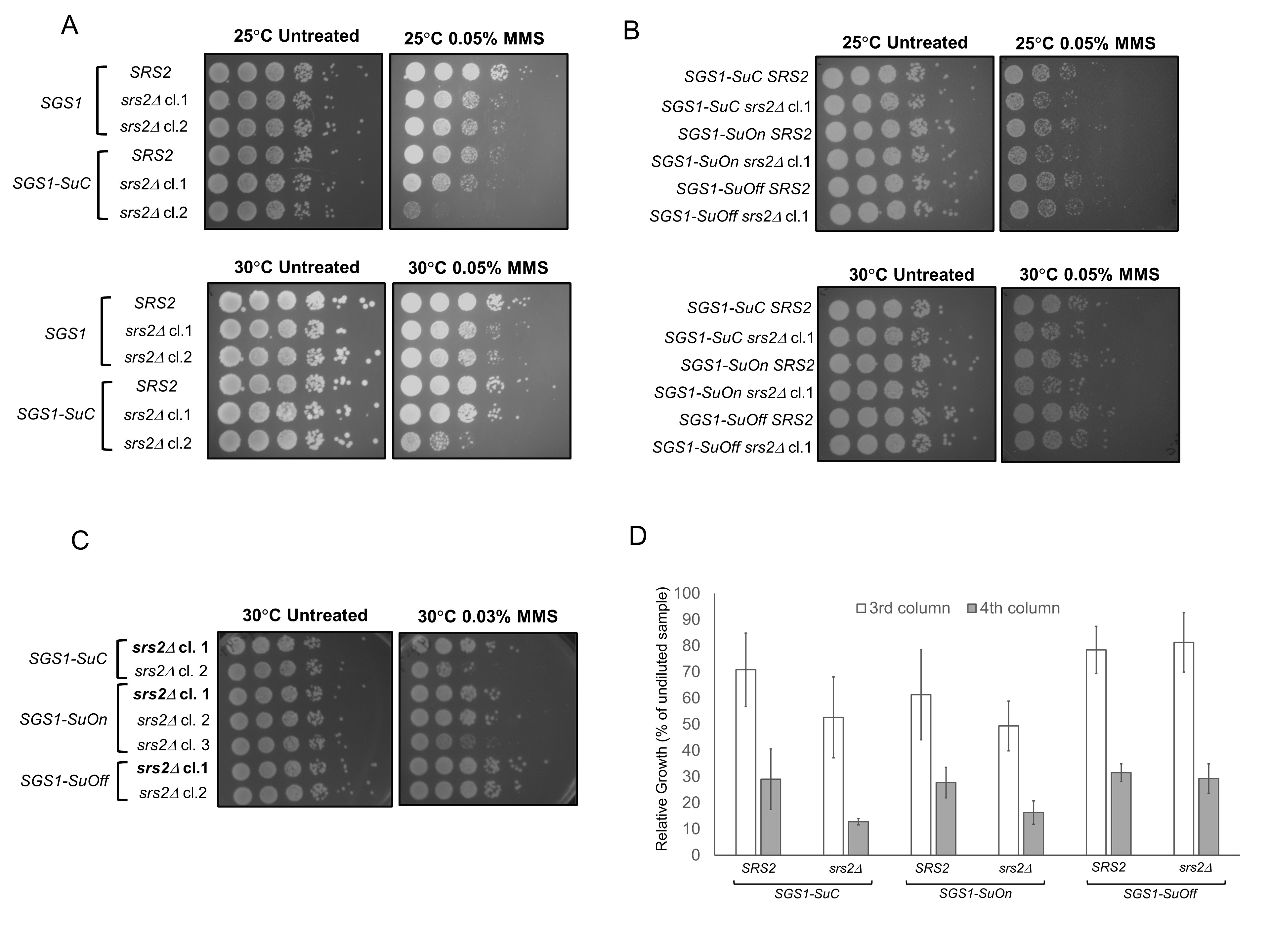
Based on these data, we asked if SGS1 SUMO mutants, similar to sgs1 knock out cells, would exhibit genetic interaction with srs2 mutants. To this end, we genetically modified SGS1 at the endogenous locus to create a condition in which Sgs1p cannot be sumoylated (SGS1-SuOff). In this strain, SGS1 gene is fused to the domain of Ulp1p, harboring the region where SUMO contacts with Ulp1p and the isopeptidase enzymatic activity. The enzymatic activity of this domain deconjugates SUMO from the target protein, preventing Sgs1p from being sumoylated (Almedawar et al. 2012; Bermúdez-López et al. 2016; Wei and Zhao 2016). This tag indeed reduces the sumoylation of Sgs1p (Bermúdez-López et al. 2016). We also generated a strain to mimic constant sumoylation of Sgs1p (SGS1-SuOn). This strain carries a transgene encoding for Sgs1p fused to the Ulp1p isopeptidase domain that retains its affinity for SUMO but harbors a mutation that renders it catalytically dead (Almedawar et al. 2012; Wei and Zhao 2016). This genetic modification is known to promote sumoylation of the protein product of the target gene likely due to an increase in local concentration of SUMO (Wei and Zhao 2016). Increase in sumoylation of the target when fused to the SuOn tag has been demonstrated with Scc1p and Mcm6p (Almedawar et al. 2012; Wei and Zhao 2016). As a control, a similar tag with point mutations that disrupt the respective function of the SUMO-contact region and the isopeptidase activity was used (SGS1-SuC) (Almedawar et al. 2012; Wei and Zhao 2016). The sumoylation status of SGS1-SuOn and SGS1-SuC has not been examined in our study.
Wild-type SGS1 and SGS1-SuC strains grew at a comparable rate and showed similar sensitivity to 0.05% MMS, suggesting that the SUMO tag does not significantly impair the growth of wild-type cells (Figure 1A). Compared to the SGS1-SuC strains, the SUMO mutants (SGS1-SuOn and SGS1-SuOff) did not exhibit appreciable MMS sensitivity (Figure 1B). Consistent with our data, Bermúdez-López et al. observed a lack of MMS sensitivity for sgs1 mutants in which three lysine residues where Sgs1p is sumoylated are converted to arginines (Bermúdez-López et al. 2016). Lack of MMS sensitivity does not preclude the possibility that Sgs1p sumoylation contributes to its function. In fact, sumoylation of Sgs1p mediates its function in the context of the STR complex and discourages crossover events during double-stranded break repair (Bermúdez-López et al. 2016). To further understand the contribution of Sgs1p sumoylation, we took a genetic approach and studied genetic interaction between SGS1 SUMO mutants and srs2. Previous studies suggest that srs2Δ is synthetically lethal with sgs1Δ (Gangloff et al. 2000). To understand if a similar genetic interaction exists between srs2 mutants and SGS1 SUMO mutants, we knocked out SRS2 in strains with wild-type SGS1 or SGS1 fused with different SUMO tags. SGS1-SuC srs2Δ cl.1 exhibits MMS sensitivity similar to that of srs2Δ clones (cl.1 and 2) (Figure 1A). However, this phenotypic similarity was not observed for SGS1-SuC srs2Δ cl.2 and therefore, this clone was excluded from later experiments (Figure 1A). We generated independent clones of SGS1-SuOn srs2Δ and SGS1-SuOff srs2Δ and chose the clones that showed consistent phenotypes for downstream experiments (Figure 1C). SGS1-SuOn srs2Δ double mutants exhibited similar MMS sensitivity compared to SGS1-SuC srs2Δ cells (Figure 1B). However, SGS1-SuOff allele suppressed the weak MMS sensitivity that srs2Δ mutants exhibited (Figures 1B, C and D).
We reason that previously reported genes that exhibit a positive genetic interaction with srs2Δ may provide insights into the function of Sgs1p sumoylation. For example, disabling the function of Rad51p rescues the sensitivity of srs2Δ mutants to MMS, UV and γ-radiation, suggesting that srs2Δ cells accumulate Rad51p-dependent recombination structures (Aboussekhra et al. 1992). Srs2p can indeed disrupt Rad51p presynaptic filaments to regulate unscheduled recombination (Krejci et al. 2003; Veaute et al. 2003). Since SGS1-SuOff mutation also rescues the weak sensitivity of srs2Δ observed in this strain, one simple explanation is that deficiency to sumoylate Sgs1p prevents or attenuates unregulated Rad51p-mediated recombination in srs2Δ cells. The phenotype of SGS1-SuOff srs2Δ can be confirmed by using another SUMO mutant of Sgs1p (sgs1-K612R or sgs1-3KR). Furthermore, knocking out RAD51 in SGS1-SuOff srs2Δ can also elucidate if Rad51p is responsible for the observed phenotype.
Methods
Request a detailed protocolGeneration of Yeast strains
All strains are the derivatives of W303-1A and the relevant genotypes are shown in Table 1. SRS2 gene was deleted by replacement of the gene with the TRP1 auxotrophic marker amplified from pRS404 (Brachmann et al. 1998).
Strains with SGS1 gene fused to the SUMO tag (SGS1-SuC, SGS1-SuOn or SGS1-SuOff) at the endogenous locus were generated using a two-step PCR-mediated integration (Tong and Boone 2006). Briefly, plasmids carrying the Ulp1 domain (amino acids 418-621) were acquired from the X. Zhao lab (Memorial Sloan Kettering). These tags have been previously described (Almedawar et al. 2012; Wei and Zhao 2016). The plasmids were used as templates to generate the first PCR product carrying the SUMO tag. The first PCR product contains 40 base pairs immediately before the stop codon of SGS1 followed by the 5’ sequence of the SUMO tag. The first product was created using the following primers: AGGTTTTAG AAATTACCGAGGTCACTACAGAGGAAGAAAGGGTAAACCTATACCTAATCC and GTATGGTGCACTCTCAGTACAATCTCTATTTTAAAGCGTCGGTTA. The second PCR product contains the overlapping region with the first product and the URA3 selectable marker. The second product was generated using the following primers: AGATTGTACTGAGAGTGCACCATAC and GTGTCGTAGTTATAAGTAACACTATTTATTTTTCTACTCTCTGTGCGGTATTTCACACCG. Two PCR products were purified, combined and annealed before being transformed into the intended yeast strains. Multiple clones were screened and successful integration was confirmed by PCR, followed by sequencing.
Measuring growth and MMS sensitivity using serial dilutions
Appropriate strains were grown overnight in YPD. From these cultures, approximately 1x 107 cells were taken based on the OD600 readings and centrifuged. The cell pellets were resuspended in 300μl of sterile water and this sample served as the undiluted stock. Serial 10-fold dilutions were then performed in a 96-well plate using sterile water. Cells were transferred to YPD or YPD plates containing MMS using a multi-pronged spotting manifold. Strains were grown for approximately 3-4 days at indicated temperatures. Experiments were repeated three times to ensure reproducibility. Quantification of data from different experiments was performed using ImageJ. Data represent percentage of growth: (growth of a specific dilution/growth of undiluted sample)x100.
Reagents
Yeast Strains
The following strains were derived from W303-1A (MATa ura3-1 ade2-1 his3-11,-15 leu2-3,-112 can1-100 trp1-1) acquired from the Bielinsky lab.
| Strain Name | Genotype | Source |
| ABy014 | W303-1A | Bielinsky lab |
| y9 | SGS1:SGS1-SuOn (URA3) | This study |
| y12 | SGS1:SGS1-SuOff (URA3) | This study |
| y15 | SGS1:SGS1-SuC (URA3) | This study |
| y42 | SGS1:SGS1-SuC (URA3) srs2::TRP1 cl.1 | This study |
| y43 | SGS1:SGS1-SuC (URA3) srs2::TRP1 cl.2 | This study |
| y44 | SGS1:SGS1-SuOn (URA3) srs2::TRP1 cl.1 | This study |
| y45 | SGS1:SGS1-SuOn (URA3) srs2::TRP1 cl.2 | This study |
| y46 | SGS1:SGS1-SuOn (URA3) srs2::TRP1 cl.3 | This study |
| y47 | SGS1:SGS1-SuOff (URA3) srs2::TRP1 cl.1 | This study |
| y48 | SGS1:SGS1-SuOff (URA3) srs2::TRP1 cl.2 | This study |
| y63 | srs2::TRP1 cl.1 | This study |
| y64 | srs2::TRP1 cl.2 | This study |
Acknowledgments
We would like to thank Dr. X. Zhao for sharing the plasmids carrying SUMO tags. We also would like to acknowledge Adam Cook and Haley Stabile for technical assistance.
References
Funding
This research was funded by Mentored Advanced Project summer research, Grinnell College to BHH and the startup fund from Allegheny College to YMT.
Reviewed By
AnonymousHistory
Received: June 21, 2021Revision received: September 2, 2021
Accepted: September 22, 2021
Published: September 24, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Herce-Hagiwara, B; Thu, YM (2021). SGS1-SuOff rescues the mild methylmethane sulfonate sensitivity of srs2Δ cells in Saccharomyces cerevisiae. microPublication Biology. 10.17912/micropub.biology.000480.Download: RIS BibTeX