Department of Genetics, ELTE Eötvös Loránd University, Budapest, Hungary
Abstract
Cell behaviors such as survival, proliferation, and death are governed by a multitude of cues, both intrinsic and extrinsic. To test whether a wild-type environment could encourage the survival and/or differentiation of neuronal progenitor cells with impaired cell cycle progression, we transplanted cells from cdk1, dtl, slbp, fbxo5, ahctf1, gins2, hdac1, mcm5, ssrp1a, and rbbp6 mutant zebrafish embryos into wild-type embryos, creating chimeric zebrafish with mutant cells in the developing eye. We found that when cells from cdk1, dtl, slbp, gins2, mcm5, or rbbp6 mutants were transplanted into wild-type hosts, survival and/or differentiation was almost always compromised in a manner consistent with cell-autonomous cell death. Interestingly, we observed that fbxo5, ahctf1, hdac1, or ssrp1a mutant cells survived and sometimes exhibited signs of differentiation when grafted into wild-type eyes.
Description
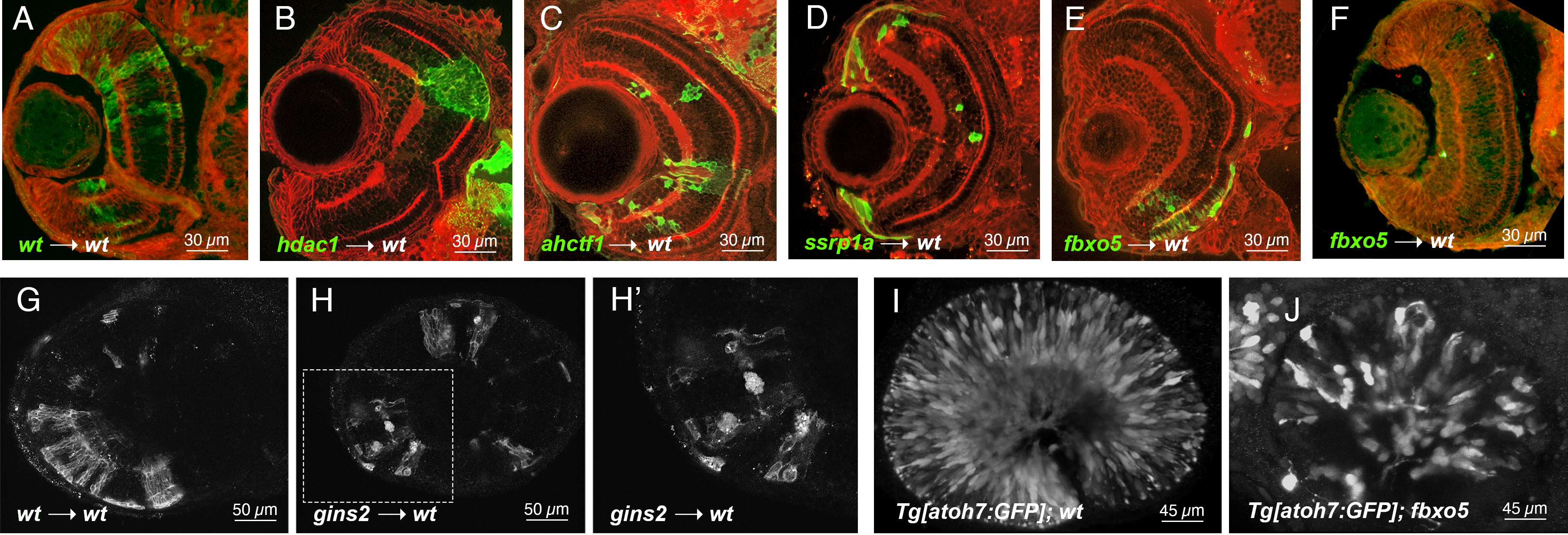
Cell behaviors such as survival, proliferation, and death are governed by a bevy of cues, both from within the cells themselves and from the local tissue environment. To test whether a wild-type environment could encourage the survival and/or differentiation of neuronal progenitor cells with impaired cell cycle progression, we created chimeric zebrafish embryos containing mutant cells in wild-type retinae. We transplanted 10-20 cells from blastula stage donors into the region of early gastrula stage wild-type hosts fated to be eye field. Donor and host embryo pairs were cultured together; donors were genotyped when possible at 1 day post fertilization (dpf). We screened all transplants at 1 dpf for survival and location of clones. Hosts that contained labeled clones in their eyes were fixed at 3 dpf and either cryosectioned before immunostaining or subjected to immunostaining as wholemounts. All embryos analyzed in this study were imaged with epifluorescence and/or confocal microscopy. Consistent with previous reports, we found that cdk1, dtl, slbp, fbxo5, ahctf1, gins2, hdac1, mcm5, ssrp1a, and rbbp6 mutant retinae contained dying cells with pyknotic nuclei throughout the developing retinal neuroepithelium at 3 dpf. Moreover, all of these mutant embryos had eyes that were noticeably smaller than their wild-type siblings (Table 1; references therein). When cells from mutant embryos were transplanted into wild-type hosts, survival and/or differentiation was almost always compromised in a manner consistent with cell-autonomous cell death. In particular, wild-type hosts that contained clones of cdk1, dtl, mcm5, and rbbp6 mutant cells at 1 dpf rarely contained visible clones by 3 dpf (see Table 1 for numbers of chimeras analyzed and how many clones survived until 3 dpf ). For those clones that were visible, they were small (e.g., compare Fig 1A and 1F) and/or exhibited signs of apoptosis (e.g. Fig 1H). For example, by transplanting gins2 morphant cells labeled with a membrane-targeted red fluorescent protein into wild-type embryos, we observed mutant cells blebbing and/or fragmenting when integrated into wild-type retinae (Fig 1H) whereas wild-type sibling cells integrated fully into the host environment, highlighting typical retinal neuronal morphologies (Fig 1G).
Clear evidence of mutant cell survival and/or differentiation was found in chimeric retinae containing ahctf1, ssrp1a, or fbxo5 homozygous mutant cells in wild-type host retinae (Fig. 1C-E, Table 1). As we previously reported, we confirmed that ahctf1 mutant cells transplanted into wild-type eyes appeared to survive and differentiate (Fig 1C, n = 19 transplants; (Cerveny et al. 2010)). The protein encoded by the ahctf1 gene (also known as elys) has been implicated in a number of cell-cycle related functions including kinetochore assembly and nuclear pore assembly (e.g., (Rasala et al. 2006)). We reasoned that mutations in other ancillary cell-cycle proteins may also be susceptible to survival and/or differentiation cues in the wild-type retinal environment. To test this hypothesis, we examined the effects of a wild-type retinal environment on ssrp1a mutant cells. The ssrp1a gene encodes a component of the conserved facilitates chromatin transcription (FACT) complex and has been implicated in cell cycle control at the level of nucleosome remodeling necessary for DNA transcription, DNA replication, and DNA repair (Liu et al. 2020). Interestingly, we observed that ssrp1a mutant cells transplanted into wild-type embryos were rarely found in the differentiated retina and almost always in the ciliary marginal zone (CMZ), a region of the retina that contains a source of stem and progenitor cells throughout the life (Fig 1D, n=10 transplants).
The fbxo5 gene encodes a protein that is both a substrate for and inhibitor of anaphase-promoting complex/cyclosome (APC/C), regulating the re-replication block, an essential step in the cell cycle (Cappell et al. 2018). Previous studies in zebrafish indicated that fbxo5 can function both cell-autonomously and non-autonomously (Riley et al. 2010) and that fbxo5 regulates genomic integrity and proliferation (Rhodes et al. 2009). We observed cell survival and differentiation in approximately one-third of fbxo5-wild-type chimeras. For instance, fbxo5 mutant cells survived and appeared to differentiate in 10/34 transplants (Fig 1E). Interestingly, the majority (8/10) of chimeric retinae with surviving and differentiating fbxo5 mutant cells were located in the ventral retina. In the remaining approximately two-thirds of our sample, however, fbxo5 mutant clones appeared to be lost by cell death and/or not differentiate in wild-type environments (Fig 1F). These data suggest that these mutant cells might be especially sensitive to slight differences in age of the donor embryo at time of transplantation, differences in location of transplanted cells, or stochastic fluctuations in gene expression (e.g.,(Trimarchi et al. 2008)) in the transplanted cells or host embryos. Because multiple people performed these transplants, it is also possible that some of the variability we observe is due to batch effects and individual technique.
Previous reports have shown that zebrafish embryos carrying homozygous mutations in fbxo5 (also known as emi1) still exhibit some neuronal differentiation (Zhang et al. 2008; Riley et al. 2010). We found that a small, but notable fraction of fbxo5 mutant retinal progenitor cells still express the neurogenic gene atoh7, as observed with the atoh7:GFP transgene (Poggi et al. 2005) and form some retinal ganglion cells (compare Fig 1I-J). The same has been shown for ahctf1 mutants (Davuluri et al. 2008; Cerveny et al. 2010) and ssrp1a mutants (Koltowska et al. 2013). It is possible, therefore, that the effects of the wild-type environment on fbxo5, ahctf1 , or ssrp1a deficient cells result from stochastic expression of some early neurogenic genes that prime cells for survival and/or differentiation in the neural retina.
Finally, our transplant studies also confirmed previous reports that mutations in histone deacetylase 1, hdac1, are linked to cell autonomous hyperproliferation in the retina (Stadler et al. 2005; Yamaguchi et al. 2005, Fig 1B). When we examined hdac1 mutant cells that had integrated into wild-type chimeric retinae at 3 dpf, a point at which apoptotic cells are found scattered throughout the hdac1 mutant retinae (Yamaguchi et al., 2005), we did not observe pyknotic nuclei or cell blebbing, two key hallmarks of apoptosis. Instead, we observed large clones that interrupted retinal lamination and did not exhibit neuronal morphologies (e.g., Fig 1B). This finding raises the possibility that a wild-type retinal environment supports the survival of these proliferative cells but does not promote their cell cycle exit and/or differentiation.
The difference in susceptibility of mutant cells to the wild-type environment may be explained, in part, by the distinct functions of the mutated genes. Of note, the only mutant cells that significantly survived and/or differentiated in a wild-type environment (ahctf1, ssrp1a, and hdac1) carry mutations in genes that impact cell cycle progression but are not part of the canonical cell cycle machinery. We speculate that mutations in genes that are not directly linked to the cell cycle but nonetheless exhibit cell cycle defects may be part of a redundant regulatory network and therefore are more likely to respond to survival and differentiation factors in a wild-type environment.
Methods
Request a detailed protocolZebrafish lines
Eggs were collected by natural spawning, raised at either 25˚C or 28.5˚C in E3 embryo medium (Nüsslein-Volhard, C. and Dahm, R. 2002) and staged according to Kimmel et al., 1995. After gastrulation and before 24 hours post-fertilization, embryos were cultured in 0.003% phenylthiourea (PTU, Sigma) in E3 to prevent pigment formation. Lines used in this study and associated references are listed in Table 1. Adult zebrafish were cared for with protocols approved by the Reed College IACUC.
Cell transplants
Similar to previously published studies (e.g., (Cerveny et al. 2010; Turner et al. 2019), donor embryos were injected at the 1-cell stage with ~20 ng of GFP mRNA synthesized from linearized pCS2-GFP or membrane-targeted RFP mRNA synthesized from linearized pCS2-membrane-targeted mCherry with the T7 mMessage mMachine kit (Ambion) according to manufacturer’s instructions. Host and donor embryos were grown at 28.5˚C until sphere stage (approximately 4 hours post-fertilization) and then 10-20 fluorescently labelled cells were removed from donor embryos and transplanted into the animal pole of unlabeled host embryos. Donor and host embryos were incubated overnight at 28.5˚C. All embryos were screened and E3 was exchanged for PTU in E3. Donors were identified by visual inspection and by PCR and restriction digest mediated genotyping. Genotyping protocols for each line can be found at Zebrafish International Resource Center (ZIRC; http://zebrafish.org/home/guide.php) and in relevant references (see Reagents; Table 1). For gins2 experiments, 1-cell stage embryos were first injected with ~1 nl of 1 mM gins2 morpholino (Gene Tools, Philomath, OR; 5’-GGGGTGAGTCAATTTATAATCTAC-3’), a dose that phenocopies gins2-/- mutants (Varga et al. 2020) and then injected with ~10 ng of membrane-targeted RFP mRNA
Immunohistochemistry, imaging, and analysis
After fixation, wholemount embryos were either subjected to immunohistochemistry as previously described (Cerveny et al. 2010) or were cryoprotected in 15% and then 30% sucrose before being embedded in Optimal Cutting Temperature (OCT) resin and cut into 30 µm thick sections that were collected on charged glass slides (Polysciences, cat number: 24216) and stained with the following antibodies: beta-catenin (mouse, 1:250 dilution; Sigma, C7207); GFP (chicken, 1:250 dilution, Abcam, ab139709); RFP (rabbit, 1:500 dilution, MBL, PM005). Nuclei were counterstained with DAPI (1 µg/ml from a 1 mg/ml stock in DMSO; Sigma) or sytox orange (1:10,000 dilution, Invitrogen). All images pictured were captured on a Nikon A1+ confocal with a long working distance 25X, 1.1 NA water immersion lens or a Leica SP8 confocal with a 20x 0.8NA water immersion lens.
Reagents
| Table 1. Cell cycle mutants examined for responsiveness to a wild-type environment by chimeric analysis in zebrafish retinae. | ||||
| Mutant | Molecular function of mutated gene according to literature | Phenotype linked to cell cycle defect as reported in literature | Phenotypes of mutant cells when transplanted into WT retinae as examined by cell morphology | References |
| cdk1hi3235Tg | binds various cyclins promoting entry into S-phase and mitosis | stall in G1, G1/S, S phases, apoptosis | apoptosis (12 chimeras analyzed; only 5/12 chimeras contained small clones (1-3 cells) by 3 dpf) | (Amsterdam et al. 2004); this study |
| ssrp1as819 | component of FACT complex, remodels chromatin, functions during transcription, DNA replication and repair | arrest in S phase, apoptosis | survival in the CMZ and RPE but some quiescence and apoptosis in neural retina (10 chimeras analyzed; 10/10 chimeras contained clones by 3 dpf) | (Koltowska et al. 2013); this study |
| dtlhi3627Tg | E3-ubiquitin ligase, regulates cyclin-dependent kinase inhibitors | arrest in late S/early G2, apoptosis | apoptosis (18 chimeras analyzed; only 3/18 chimeras contained very small clones (1-3 cells) by 3 dpf) | (Sansam et al. 2010); this study |
| slbp1ty77e | binds stem-loop structure of histone mRNAs, stabilizes pre-mRNA-snRNP interactions | stall in G1/S, apoptosis | apoptosis (17 chimeras analyzed; 16/17 chimeras contained visible clones by 3 dpf) | (Turner et al. 2019); this study |
| fbxo5hi2648Tg | APC/C inhibitor, known to block re-replication | primarily arrest in G2/M, apoptosis. | some differentiation but also some apoptosis; highly variable (34 chimeras analyzed; 10/34 chimeras had visible clones by 3 dpf; 24/34 chimeras had very small (1-3 cells), but visible clones by 3 dpf) | (Rhodes et al. 2009; Riley et al. 2010; Zhang et al. 2008); this study |
| ahctf1ti262c | kinetochore protein also required for nuclear pore assembly | cycle slowly, stalling in either G1/S or G2/M | survival and differentiation (19 chimeras analyzed; 19/19 chimeras had visible clones by 3 dpf) | (Cerveny et al. 2010; Davuluri et al. 2008); this study |
| gins2u773(also used morpholinos) | DNA replication initiation and progression | Delayed/prolonged S phase, apoptosis | apoptosis (26 chimeras analyzed; 26/26 chimeras had visible clones by 3 dpf) | (Varga et al. 2020); this study |
| hdac1hi1618Tg | removes acetyl groups linked to lysine residues typically found on histones | unable to exit the cell cycle; slowly proliferate and do not differentiate | survival and proliferation (14 chimeras analyzed at 4 dpf; 9 chimeras analyzed at ~3 dpf; all contained visible clones) | (Yamaguchi et al. 2005; Zhou et al. 2011); this study |
| mcm5m850 | component of a DNA helicase, required during S-phase | prolonged S phase, apoptosis | apoptosis (15 chimeras analyzed; only 10/15 chimeras contained very small clones (1-3 cells) by 3 dpf) | (Ryu et al. 2005); this study |
| rbbp6hi2993Tg | E3-ubiquitin ligase with functions linked to DNA replication and DNA repair | predicted to arrest in G1/S, apoptosis | apoptosis (12 chimeras analyzed; only 1/12 chimeras contained very small clones (1-3 cells) by 3 dpf) | (Amsterdam et al. 2004); this study |
| Tg[atoh7:GFP]rw021Tg | labels progenitors as they are being specified as retinal ganglion cells | (Poggi et al. 2005) | ||
Acknowledgments
The authors would like to thank Steve Wilson and current and former members of the Wilson lab who provided critical input when this study was being conceptualized.
References
Funding
NIH grant 1R15EY023745-01 to KLC, an instrumentation grant to KLC from the MJ Murdock Trust, and start-up funds from Reed College. MV was supported by the ELTE Institutional Excellence Program (1783-3/2018/FEKUTSRAT) sponsored by the Hungarian Ministry of Human Capacities.
Reviewed By
AnonymousHistory
Received: June 26, 2021Revision received: September 22, 2021
Accepted: September 22, 2021
Published: October 1, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Cerveny, KL; Bronstein, H; Hagen, O; Lamb, DB; Martin, G; Tower, I; Van Duzer, A; Welch, E; Varga, M (2021). Mutations linked to loss of cell cycle control can render cells responsive to local differentiation cues. microPublication Biology. 10.17912/micropub.biology.000481.Download: RIS BibTeX