Graduate Program in Chemistry, Facultad de Química, Universidad de la República, Uruguay.
Phylumtech S.A. Belgrano 758, Sunchales, Santa Fe, Argentina.
Laboratorio de Química Farmacéutica, Departamento de Química Orgánica, Facultad de Química, Universidad de la República, Uruguay
Laboratorio de Química Farmacéutica, Departamento de Química Orgánica, Facultad de Química, Universidad de la República, Montevideo, Uruguay
Worm Biology Laboratory, Institut Pasteur de Montevideo, Mataojo 2020, Montevideo 11400, Uruguay
Departamento de Biociencias, Facultad de Química, Universidad de la República, Avda. Gral Flores 2124, Montevideo 11300, Uruguay
Abstract
Parasitic nematodes constitute a health problem for humans, livestock and crops, and cause huge economic losses to developing-country economies. Due to the spread of nematicide resistance, there is an urgent need for new drugs. C. elegans is now recognized as a cost-effective alternative for the screening of compound libraries with potential nematicidal activity, as parasitic organisms are hard to maintain under laboratory conditions. Here we describe an adaptation of a previously reported high throughput (HTP) infrared-based motility assay that leads to increased sensitivity. The modified assay uses L1 instead of L4 stage worms and matches the sensitivity reported by Burns et al. (2015) for the anthelmintic benzamides Wact11 and Wact11p. In addition, this method presents practical advantages over Burns et al. (2015) and other image-based protocols and provides a robust assay with a fast and simple readout ideal for HTP drug discovery.
Description
Nematodes are a diverse phylum that includes numerous lineages that parasitize humans, animals, and terrestrial plants, being a health threat to humans, livestock and crops that heavily impacts food production and economies, particularly in developing countries. The main control strategy for nematode-caused infections relies on chemotherapy, but there are few compound families available and their efficacy is seriously compromised due to resistance dissemination, chiefly in crops and livestock. This demands an urgent need for new nematicides to be used in field applications. (Caffrey et al. 2012)
One of the main obstacles to designing and discovering new nematicidal agents is the lack of efficient screening assays to assess the activity of compound libraries. The free-living nematode Caenorhabditis elegans, a widely used model in biology, is currently recognized as a cost-effective alternative HTP of either synthetic or natural product libraries (Salinas and Risi 2018).
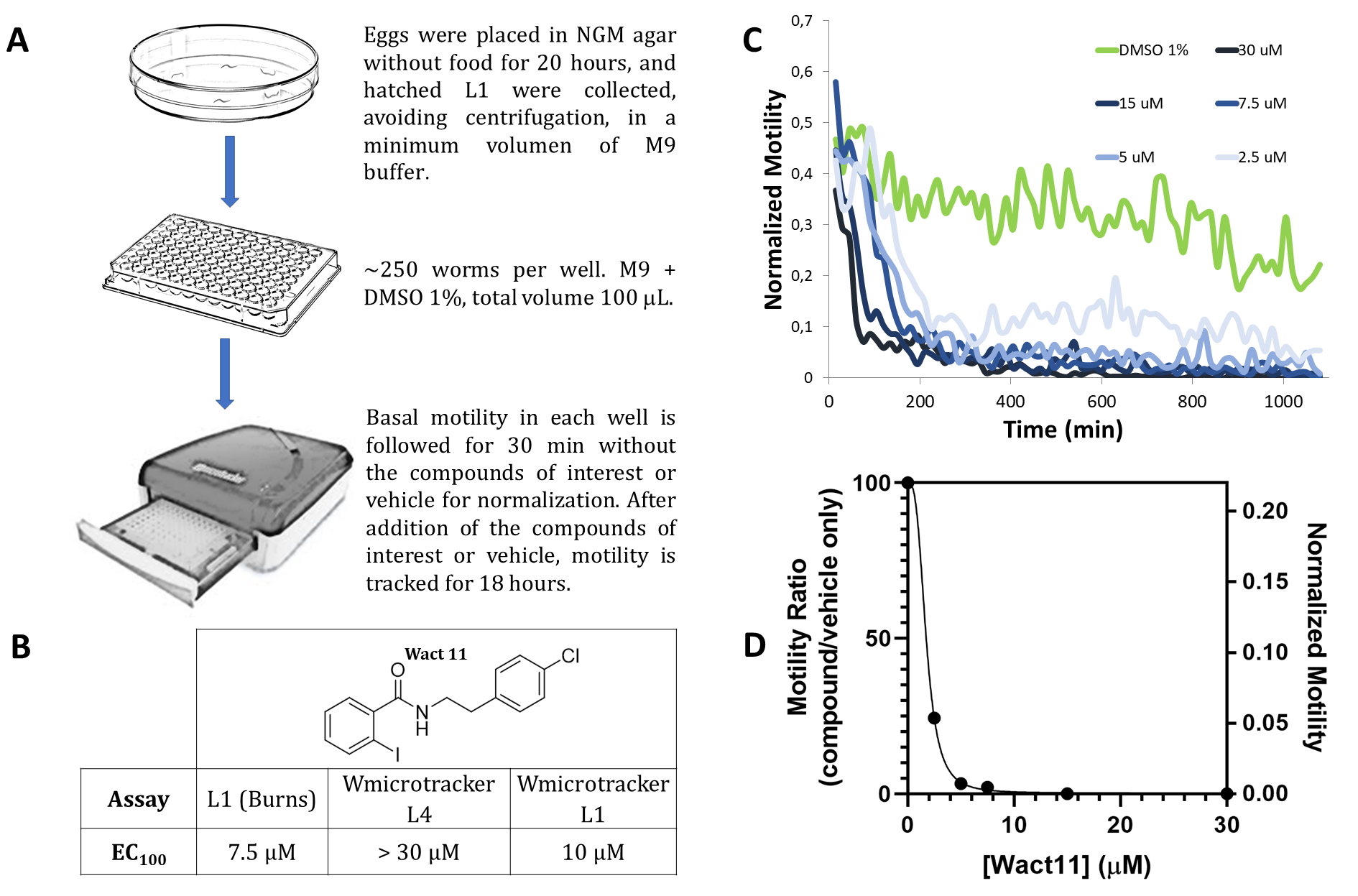
Within the framework of a nematicide discovery project, we initially took advantage of a previously described infrared-based motility method using C. elegans L4 larvae and the WMicrotracker device, (Gunderson et al. 2020) which automatically monitors the motility of the worms as a vitality proxy. The assay allowed to screen both natural product and synthetic libraries and was validated with commercial nematicides. (Risi et al. 2019). However, when Wact11 and Wact11p, two members of the nematicidal benzamide family, were assayed through this method, we found a notable discrepancy between the activity values previously reported for these compounds and those obtained with WMicrotracker, being the apparent potency much lower in the latter. Burns et al. report a LD100 of 7.5 µM for both Wact11 and Wact11p. In the infrared-based assay, a total death/paralysis phenotype was not observed even at 30 µM, the highest concentration tested (Figure, panel B). Furthermore, when applied to an in-house synthesized chemolibrary, the original L4 assay precluded us from extracting information about the structure-activity relationships of derivatives, as all of them were indistinguishable from the vehicle control. This compromised the utility of the assay to guide the design of new derivatives.
To understand the difference and overcome this obstacle, we examined in detail the protocol developed by Burns et al. in comparison with the WMicrotracker assay. The main differences are the larval stage employed, the assay time, the monitoring method and the presence of E. coli as a food source during incubation with tested compounds.
We reasoned that the crucial difference between both assays was the worm larval stage. L1 larvae could be inherently more sensitive to xenobiotic aggression than L4 due to cuticle permeability (Hartman et al. 2021) or to metabolic differences. Thus, to increase the sensitivity of the infrared-motility assay we used a direct adaptation of the original L4 protocol using L1 larvae synchronized in liquid medium, keeping all other assay parameters constant. However, this change did not allow reasonable basal motility to be detected, even increasing the number of larvae per well up to 1000. We thus explored additional modifications to the original protocol, letting L1 larvae synchronize overnight without any food source in an NGM agar plate, in order to increase their basal motility. Approximately 250 of these starved L1 larvae per well in M9 + BSA 0.015% are enough to reliably detect their movement by the WMicrotracker device. Assay time, vehicle and data process were all kept unchanged. Starvation on plates seems to be crucial for movement detection of L1 larvae and allowed motility to be measured over 18 hours. For nematicide discovery this is a potential advantage, but it should be born in mind that starvation alters gene expression and metabolism, and therefore initial screens should be followed by experiments under other conditions.
When the infrared L1-adapted assay was employed to assess Wact11 activity against C. elegans, an EC100 value of 10 µM was obtained. The adapted assay resulted in a significant increase in sensitivity compared to the original L4 assay, and in close agreement with the EC100 value reported by Burns et al. (7.5 µM), The dose-response curve (Figure 1, panel D) at the endpoint showed a clear dose-dependent effect for this compound in the L1 assay. Importantly, the adapted assay features several advantages compared to Burns et al. protocol, such as reduced assay time (18 h vs. several days), the absence of E. coli during compound incubation (which could interfere by absorbing and metabolizing the compound being tested) and microscopy-independent automatized monitoring, which significantly increased throughput and efficiency. This protocol is thus a valuable alternative when testing compound libraries with nematicidal potential. Furthermore, the modifications made to the infrared motility assay could be applied to other protocols as a way to modulate sensitivity/specificity tradeoff when using C. elegans as a drug screening model, either in medicinal chemistry or toxicology applications.
Methods
Request a detailed protocolWact11 and Wact11p synthesis
Under N2 atmosphere, 2-(trifluoromethyl)benzoic acid (for Wact11) or 2-iodobenzoic acid (for Wact11p) (1.1 mmol) was dissolved in dry CH2Cl2 (2 mL), then oxalyl chloride (1.6 mmol) and DMF (0.02 mL) were added dropwise. The mixture was stirred for 10 minutes at room temperature and then the solvent was removed under reduced pressure. The residue was redissolved in CH2Cl2 (2 mL) cooled into an ice bath followed by the addition of Et3N (1.1 mmol) and 2-(4-chlorophenyl)ethan-1-amine) (1.1 mmol). After 4 hours, the mixture was poured into water and was extracted with AcOEt. The combined organic layers were washed with HCl 5% and NaHCO3 10% and dried with Na2SO4. The solvent was removed under reduced pressure and the crude was purified by silica column chromatography (CH2Cl2/Hx (3:1)) to yield Wact11 (58%) or Wact11p (76%) as a white solid. Spectroscopic data is in concordance with product structure.
C. elegans culture
Wild-type C. elegans Bristol strain N2 and E. coli OP50 strain were obtained from the Caenorhabditis Genomics Center (Minneapolis, MN, USA). The worms were maintained under standard conditions at 20 °C on nematode growth media (NGM) agar plates seeded with E. coli OP50 as a source of food. The wild-type C. elegans Bristol strain N2 was cultured and maintained according to the procedures described previously (Brenner, 1974).
L4 assay
The L4 assay was conducted followed the protocol described in Risi et al. 2019.
L1 assay
Eggs obtained from bleaching were placed on empty magnesium complemented NGM plates (without bacteria) and let hatch for 20 hours at 20°C. L1 worms were collected in a minimum volume of M9 buffer. Approximately 250 worms were seeded in each well of a microtiter plate in 80 microliters of M9 buffer with 0.015 % BSA. Motility was tracked for 30 min. in the WMicrotracker device for normalization. After this, compounds of interest in vehicle (M9 + DMSO 1%) or vehicle alone were added in a volume of 20 microliters. Motility was tracked for additional 18 hs. Counts per well were normalized with the value obtained previously to compound or control addition.
Reagents
Bristol N2 strain of C. elegans, available at CGC.
2-(trifluoromethyl)benzoic acid, 2-iodobenzoic acid and oxalyl chloride (Sigma-Aldrich). Triethylamine (Merck), 4-chlorophenyl)ethan-1-amine (Accela). Solvents were freshly distilled before the reaction.
References
Funding
ANII graduate scholarship POS_NAC_D_2020_1_164031, CAP graduate scholarship, CSIC, FOCEM - Fondo para la Convergencia Estructural del Mercosur (COF 03/11).
Reviewed By
AnonymousHistory
Received: September 10, 2021Revision received: November 3, 2021
Accepted: November 16, 2021
Published: November 30, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Vairoletti, F; Baron, A; Saiz, C; Mahler, G; Salinas, G (2021). Increased sensitivity of an infrared motility assay for nematicide discovery. microPublication Biology. 10.17912/micropub.biology.000500.Download: RIS BibTeX