Abstract
Many of the Drosophila enzymes involved in carbohydrate metabolism are coordinately up-regulated approximately midway through embryogenesis. Previous studies have demonstrated that this metabolic transition is controlled by the Drosophila Estrogen-Related Receptor (dERR), which is stabilized and activated immediately prior to onset of glycolytic gene expression. The mechanisms that promote dERR activity, however, are poorly understood and other transcriptional regulators could control this metabolic transition, independent of dERR. In this regard, the steroid hormone 20-hydroxyecdysone (20E) represents an intriguing candidate for regulating glycolytic gene expression in embryos – not only does the embryonic 20E pulse immediately precede transcriptional up-regulation of glycolytic metabolism, but 20E is also known to promote Lactate dehydrogenase gene expression. Here I test the hypothesis that embryonic 20E signaling is required to activate glycolytic gene expression. Using developmental northern blots, I demonstrate that the transcriptional up-regulation of glycolytic genes during embryogenesis still occurs in shadow mutants, which are unable to synthesize either ecdysone or 20E. My finding indicates that ecdysone and 20E signaling are not required for this mid-embryonic metabolic transition.
Description
During the course of the Drosophila development, metabolism readily adapts to meet the energetic and biosynthetic demands of each life stage (Gillette et al., 2021). This relationship between growth and metabolism is particularly apparent when examining changes in glycolytic gene expression. Approximately midway through embryonic development, transcripts representing nearly every enzyme involved in glycolysis are coordinately up-regulated (Abu-Shumays and Fristrom, 1997; Currie and Sullivan, 1994a, b; Madhavan et al., 1972; Roselli-Rehfuss et al., 1992; Shaw-Lee et al., 1992; Shaw-Lee et al., 1991; Sun et al., 1988; Tennessen et al., 2011; Tennessen et al., 2014; Tixier et al., 2013; Wright and Shaw, 1970). The resulting glycolytic program is maintained throughout the larval growth period and subsequently down-regulated prior to the onset of metamorphosis (White et al., 1999). These metabolic transitions are highly predictable and provide an opportunity to understand how metabolism adapts to meet the energetic demands of insect development.
Activation of glycolytic metabolism in Drosophila embryos depends on the Drosophila Estrogen-Related Receptor (dERR; FBgn0035849), which represents the sole fly ortholog of the orphan class of ERR nuclear receptors (Ostberg et al., 2003). In embryos cultured at 25ºC, dERR becomes transcriptionally active ~10-12 hours after oviposition, resulting in the coordinate up-regulation of genes involved in carbohydrate metabolism (Tennessen et al., 2011). The mechanisms that temporally regulate dERR activity during this metabolic switch, however, remain unknown and additional factors could be controlling embryonic glycolytic metabolism independent of dERR. In this regard, both dERR activation and onset of glycolytic gene expression correlate with the embryonic pulse of the steroid hormone 20-hydroxyecdysone (20E), which triggers dorsal closure, cuticle deposition, head involution, and a variety of other developmental events (Chavez et al., 2000; Kozlova and Thummel, 2003; Maróy et al., 1988; Warren et al., 2002). Moreover, not only is 20E known to induce Lactate Dehydrogenase (Ldh; FBgn0001258) expression in imaginal discs (Abu-Shumays and Fristrom, 1997), but the Ecdysone Receptor (EcR; FBgn0000546) and dERR are also reported to cooperatively regulate expression of glycolytic genes in insect cell culture (Kovalenko et al., 2019), thus raising the possibility that 20E-signaling is necessary for the up-regulation of embryonic carbohydrate metabolism.
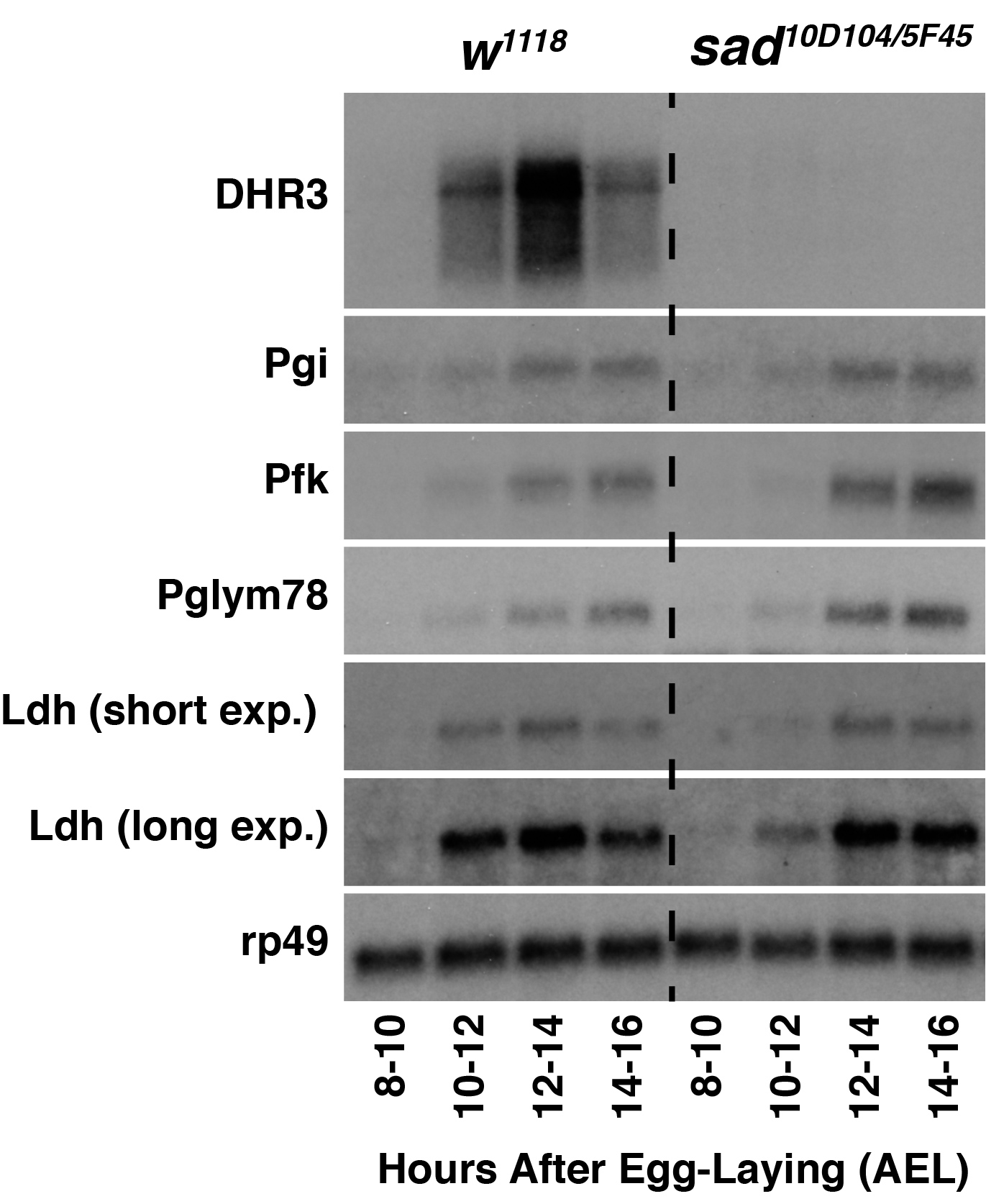
To test the hypothesis that 20E transcriptionally regulates glycolysis in Drosophila embryos, I used developmental northern blots to analyze expression of key glycolytic genes in shadow (sad; FBgn0003312) mutants, which are unable to synthesize either ecdysone or 20E (Warren et al., 2002). As a positive control, I first analyzed expression of DHR3 (FBgn0000448), which is up-regulated in response to embryonic 20E production (Ruaud et al., 2010). Unlike w1118 control samples that expressed DHR3 during mid-embryogenesis, DHR3 transcripts were undetectable in sad mutant samples – a result consistent with lack of 20E production in these embryos. In contrast, sad mutants still exhibit the coordinate up-regulation of Phosphoglucoisomerase (Pgi; FBgn0003074), Phosphofructokinase (Pfk; FBgn0003071), and Phosphoglyceromutase 78 (Pglym78; FBgn0014869) transcripts during mid-embryogenesis, indicating that 20E is not necessary to activate glycolytic gene expression during mid-embryogenesis. I would note, however, that Ldh transcripts levels were noticeably decreased at the 10-12 hour AEL timepoint in sad mutant samples when compared with controls, suggesting that 20E partially regulates Ldh expression at this early timepoint. Such a possibility is consistent with previous observations that 20E activates Ldh expression in imaginal discs and that embryonic Ldh mRNA transcript levels are elevated at an earlier timepoint when compared with other glycolytic genes (Abu-Shumays and Fristrom, 1997; Tennessen et al., 2011).
Overall, my findings suggest that that 20E signaling is not required for the coordinate up-regulation of glycolytic gene expression observed midway through embryonic development. All glycolytic genes examined in this study exhibited normal up-regulation in sad mutants, with the only exception being Ldh, which exhibited slightly delayed onset of expression when compared with the control strain. My finding, however, does not exclude the possibility that ecdysone or 20E controls glycolytic metabolism within individual cells or tissues that would be overlooked using northern blot analysis. Finally, my study further highlights the role of dERR as a central regulator of carbohydrate metabolism during mid-embryogenesis and again raises the question as to what developmental signals induce dERR activity during this metabolic transition.
Methods
Request a detailed protocolDrosophila Genetics and Embryo Collection: All strains were maintained on Bloomington Drosophila Stock Center (BDSC) media. Strains containing the sad alleles sad10D104 and sad5F45 (a kind gift from Dr. Michael O’Connor) were individually crossed to BDSC stock #6663 (w1118; DrMio/TM3, P{w[+mC]=GAL4-twi.G}2.3, P{UAS-2xEGFP}AH2.3, Sb1 Ser1). F1 male offspring were again crossed into BDSC strain #6663 and progeny lacking the DrMio chromosome were used to establish balanced stocks.
Egg collection and synchronization were conducted as previously described (Li and Tennessen, 2017). For both w1118 controls and sad mutants, 50 females and 25 males were placed in an egg-laying bottle with a molasses agar plate containing a smear of yeast paste taped in the lid. In the case of sad mutant embryo collections, sad10D104/TM3, P{w[+mC]=GAL4-twi.G}2.3, P{UAS-2xEGFP}AH2.3, Sb1 Ser1 virgin females were crossed with sad5F45/TM3, P{w[+mC]=GAL4-twi.G}2.3, P{UAS-2xEGFP}AH2.3, Sb1 Ser1 males. sad10D104/5F45 mutant embryos were identified by the absence of GFP expression using a Zeiss SterREO Discovery V8 microscope.
Northern Blots: RNA preparation and northern blot analysis was conducted using a previously described method (Karim and Thummel, 1992). Briefly, staged embryos were dechorionated and RNA extracted using Trizol Reagent (Life Technologies) following the manufacturers protocol. 3 µg total RNA from each sample was individually added to a 1.5 ml microcentrifuge tube containing a premixed solution of 3 µl 10X Formaldehyde Gel Buffer (0.2 M MOPS pH 7.0, 50 mM Sodium Acetate, 10 mM EDTA), 3.5 µl of formaldehyde (37% w/v), and 10 µl of formamide. Sample volume was adjusted to 25 µl by adding the appropriate volume of nuclease free H2O, incubated at 65ºC for 5 minutes, and centrifuged briefly at 10,000 x g. 3 µl of loading dye (80% glycerol, 1 mM EDTA, 0.4% bromophenol blue, 0.4% xylene cyanol) was added to the sample and mixed by pipetting. The entire sample was then loaded into a formaldehyde-agarose gel (1 g agarose, 75 mL H2O, 10 mL of 10X formaldehyde gel buffer, 15 mL of 37% formaldehyde [m/v]; immersed within 1X formaldehyde gel buffer). RNA samples were separated at 70 volts for 150 minutes.
RNA was transferred overnight from the gel to GeneScreen Hybridization Transfer Membrane (Perkin Elmer) using standard blotting techniques and crosslinked to the membrane using the “Autocrosslink” setting on a Stratagen UV Stratalinker 1800. Prior to hybridization, membranes were pretreated for one hour at 42ºC with 10 ml of hybridization buffer (5 ml of formamide, 2 ml of 10X PIPES buffer [0.1 M PIPES, pH6.5, 4 M NaCl], 1 ml of 10% SDS, 2 ml H20, 100 µl sheared herring sperm DNA).
For Pfk, Pgi, Pgylm78, and Ldh, radioactive probes were generated from PCR amplified cDNA fragments (see reagents table for oligos used to synthesize cDNA fragments). For DHR3 and rp49, probes were generated as previously described (Sullivan and Thummel, 2003). Labeling reactions were conducted with 32P-labeled dCTP ([a-32P]- 3000Ci/mmol 10mCi/ml EasyTide, 250 µC; Perkin Elmer; BLU513H250UC) using a Prime-It II Random Primer Labeling Kit (Agilent Catalog #300385) following the manufacturer’s instructions. Individual reactions were cleaned using a MicroSpin G-50 Sephadex column (Amersham Catalog #27533001). Radioactive probes were added to 100 µl of sheared herring sperm DNA in a 2 ml screwcap tube, boiled for 5 minutes, and added to the hybridization tube containing the membrane and buffer.
Labeled probes were allowed to hybridize with the membrane overnight at 42ºC, at which time the hybridization buffer/probe mixture was poured out of the hybridization tube and the membrane washed twice for 10 minutes at 42ºC with 10 ml of 2X SSC + 0.1% SDS, once for 10 min at 55ºC with 10 ml of 1X SSC + 0.1% SDS, and once for 10 min at 55ºC with 10 ml of 0.1X SSC + 0.1% SDS. After the final wash, the membrane was removed from the hybridization tube, briefly immersed in 2X SSC, wrapped in plastic wrap, sandwiched between two intensifying screens with a piece of film, and placed at -80ºC for 24 hours. Exposed film was developed using a Kodak X-OMAT film processor.
Reagents
Oligo sequences used to generate northern blot probes
| Gene | Oligo sequences |
| Pfk | 5’- ATGCATTCAATAAAATTTCGAGTATTTACC-3’
5’- TTAGGCGACGGCGTCAGTGTCAC-3’ |
| Pgi | 5’- ATGGCCGGCCCACTTCCTCC -3’
5’- TTACTTCCAATTGGCTTTGATG-3’ |
| Pglym78 | 5’- CCACTACGGTGGACTCACTG -3’
5’- ATGGCCTTCTTCACGGTCTC -3’ |
| Ldh | 5’- ATGGCCGCCATTAAGGACAGTCTG -3’
5’- TTAGAACTTCAGACCAGCCTGGAC -3’ |
| Strain | Genotype | Available from |
| 5905 | w[1118] | BDSC |
| JMT49 | w[1118]; sad[10D104], st, e/TM3, P{w[+mC]=GAL4-twi.G}2.3, P{UAS-2xEGFP}AH2.3, Sb[1] Ser[1] | Tennessen Lab |
| JMT54 | w[1118]; sad [5F45] ru, h, th, st, cu, sr, e/TM3, P{w[+mC]=GAL4-twi.G}2.3, P{UAS-2xEGFP}AH2.3, Sb[1] Ser[1] | Tennessen Lab |
Acknowledgments
I thank Dr. Michael B. O’Connor for providing strains containing the sad alleles, the Bloomington Drosophila Stock Center for fly stocks (NIH P40OD018537), the Drosophila Genomics Resource Center (NIH 2P40OD010949) for the cDNA construct used in our analysis, and Flybase (NIH U41HG000739).
References
Funding
J.M.T. is supported by a R35 MIRA award from the National Institute of General Medical Sciences of the National Institutes of Health (R35GM119557).
Reviewed By
AnonymousHistory
Received: September 20, 2021Revision received: November 14, 2021
Accepted: November 15, 2021
Published: November 30, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Tennessen, JM (2021). Ecdysone and 20-hydroxyecdysone are not required to activate glycolytic gene expression in Drosophila melanogaster embryos. microPublication Biology. 10.17912/micropub.biology.000501.Download: RIS BibTeX