Abstract
Valence of animal pheromone blends can vary due to differences in relative abundance of individual components. For example, in C. elegans, whether a pheromone blend is perceived as “male” or “hermaphrodite” is determined by the ratio of concentrations of ascr#10 and ascr#3. The neuronal mechanisms that evaluate this ratio are not currently understood. We present data that suggest that the function of guanylyl cyclase ODR-1 in AWB neurons is required for the effect of ascr#3 that counteracts the activity of ascr#10. This finding defines a new module in the neuronal mechanism that determines the sexual identity of C. elegans pheromone.
Description
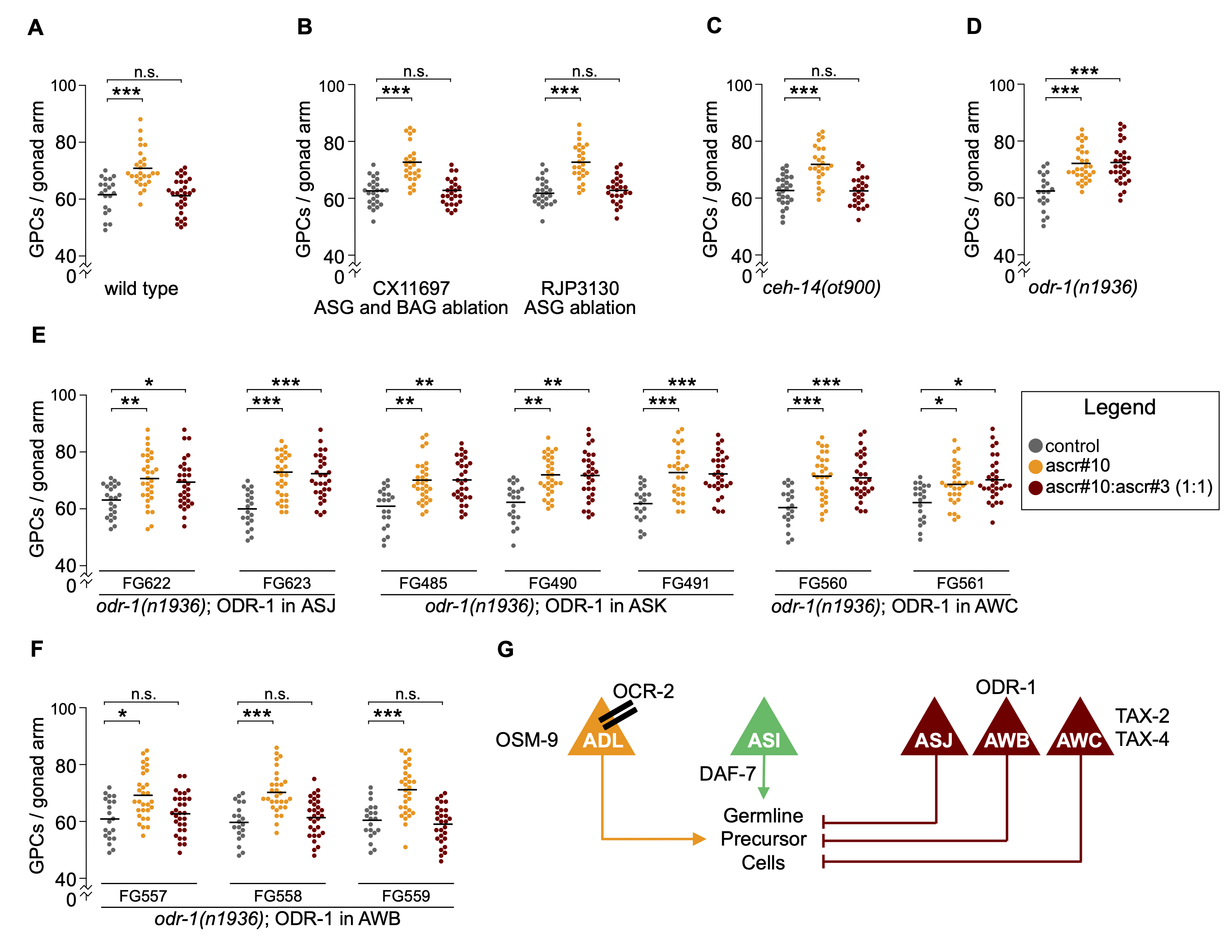
In C. elegans, complex blends of small molecules act as pheromones; the best known of these are ascarosides (Srinivasan et al., 2008; Srinivasan et al., 2012). Although hermaphrodites and males produce similar ascaroside profiles, there are several notable and functionally consequential differences. The best studied one is that males excrete blends enriched in ascr#10, whereas hermaphrodite blends are enriched in a nearly-identical (a difference of a single unsaturated bond) ascr#3 (Izrayelit et al., 2012). Previously, we showed that hermaphrodites exposed to the male pheromone have an enlarged population of germline precursor cells (Aprison and Ruvinsky, 2016). This effect can be recapitulated by physiological concentrations of synthetic ascr#10 and ascr#3 (Aprison and Ruvinsky, 2016), as long as the concentration of the male-enriched ascr#10 is higher than that of ascr#3 (Aprison and Ruvinsky, 2017). Central to discriminating between blends with different concentration ratios of these two ascarosides is that ascr#3 counteracts the effect of ascr#10, resulting in no discernable effect when the concentration of ascr#3 was greater or equal to that of ascr#10 (Aprison and Ruvinsky, 2017). Therefore, in our paradigm, a mutant strain can be tested for the ability to respond to ascr#3 only if it responds to ascr#10 (this response is manifested as an increased number of germline precursors). In the absence of ascr#10 response, ascr#3 response cannot be assessed because this compound does not change the number of germline precursors (Figure 1A).
Using this approach, we identified a set of six pairs of sensory neurons (ASG, ASI, ASJ, ASK, AWB, and AWC), at least some of which being required for the ascr#3 response (Aprison and Ruvinsky, 2017). Our previous experiments with neuron-specific ablations established that ASJ, AWB, and AWC neurons were required for ascr#3 response, whereas ASI were required for ascr#10 response, and ASK played no obvious role in our paradigm (Aprison and Ruvinsky, 2017). In the present study, using strains that ablate ASG (as well as BAG) neurons (Juozaityte et al., 2017), we found that loss of these cells does not affect hermaphrodites’ ability to respond to ascr#3 (Figure 1B). Similarly, mutants carrying a strong loss-of-function allele in a LIM homeobox gene ceh-14(ot900) (Bayer and Hobert, 2018) had a wild type ascr#3 response (Figure 1C). These results argue that response to ascr#3 does not require ASG, BAG, and ~20 neurons that express ceh-14 (Bayer and Hobert, 2018; Cassata et al., 2000; Kagoshima et al., 2013; Taylor et al., 2021).
The six pairs of sensory neurons, ASG, ASI, ASJ, ASK, AWB, and AWC, express tax-2 and tax-4 genes encoding subunits of a cyclic nucleotide-gated channel. These cells also express odr-1 (Taylor et al., 2021), a gene that encodes a receptor guanylate cyclase thought to supply cGMP to the TAX-2/TAX-4 channel (L’Etoile and Bargmann, 2000). Loss of odr-1 eliminated ascr#3 response (Figure 1D). To identify the site of odr-1 action, we used strains that narrowly expressed the wild type ODR-1 protein in the background of the loss-of-function mutant odr-1(n1936) (Krzyzanowski et al., 2016). We focused on the latter four neuronal pairs because loss of ASG does not affect ascr#3 response (Figure 1B), while ASI is involved in ascr#10 response and therefore could not be tested in this paradigm (Aprison and Ruvinsky, 2017). Expression of ODR-1 under control of trx-1 (in ASJ), srbc-66 (in ASK), or ceh-36 (in AWC) did not rescue the odr-1(n1936) mutant phenotype (Figure 1E). A comprehensive gene expression atlas detected transcripts of trx-1, srbc-66, and ceh-36 in few additional cells (PVR, AWA, and ASE, respectively); these neurons do not appear to express odr-1 (Taylor et al., 2021). Expression of ODR-1 under control of str-1 robustly rescued odr-1(n1936) (Figure 1F). str-1 transcripts were present in AWB and RIP neurons, two neuronal classes in which odr-1 was also detected (Taylor et al., 2021). Because no ODR-1 function in RIP has been described, whereas AWB is a major site of ODR-1 activity (L’Etoile and Bargmann, 2000), we currently favor the hypothesis that, with respect to response to ascr#3, ODR-1 acts in AWB neurons.
It is not currently clear whether ODR-1 in AWB is involved in sensing ascr#3. Although AWB are amphid sensory neurons, they are generally thought to sense volatile repellents (Bargmann, 2006), whereas ascarosides do not appear to be volatile. It is possible that ODR-1 in AWB modulates activity of an ascr#3-sensing neuron(s) or is required further downstream for the germline response to ascr#3. Because our paradigm relied on scoring the population of germline precursor cells, we conservatively conclude that all neurons and gene products identified so far are involved in “the germline response to ascr#10 and ascr#3”, although we assume that at least some of these are required for sensing the two sex pheromones and comparing their concentrations. The current state of knowledge of the sensory components involved in the germline response to ascarosides is summarized in Figure 1G.
Methods
Request a detailed protocolWe used standard C. elegans methods as previously published (Aprison and Ruvinsky, 2017, 2019a, b). Additional protocol details are available upon request.
Reagents
| Strain | Genotype | Available from |
| N2 | wild type | CGC |
| CX11697 | kyIs536[flp-17::p17::SL2::GFP, elt-2::mCherry] | Bargmann lab |
| RJP3130 | otEx3900 [ops-1p::p12; gcy-21p::p17; myo-3p::RFP]; rpEx1523[ets-5p::mCherry; elt-2p::GFP] | Pocock lab |
| OH15422 | ceh-14(ot900) | CGC |
| CX2065 | odr-1(n1936) | CGC |
| FG622 | odr-1(n1936); udEx232[trx-1p::odr-1; elt-2p::gfp] | Ferkey lab |
| FG623 | odr-1(n1936); udEx231[trx-1p::odr-1; elt-2p::gfp] | Ferkey lab |
| FG485 | odr-1(n1936); udEx276[srbc-66p::odr-1; elt-2p::gfp] | Ferkey lab |
| FG490 | odr-1(n1936); udEx214[srbc-66p::odr-1; elt-2p::gfp] | Ferkey lab |
| FG491 | odr-1(n1936); udEx215[srbc-66p::odr-1; elt-2p::gfp] | Ferkey lab |
| FG560 | odr-1(n1936); udEx307[ceh-36p3::odr-1; elt-2p::gfp] | Ferkey lab |
| FG561 | odr-1(n1936); udEx308[ceh-36p3::odr-1; elt-2p::gfp] | Ferkey lab |
| FG557 | odr-1(n1936); udEx304[str-1p::odr-1; elt-2p::gfp] | Ferkey lab |
| FG558 | odr-1(n1936); udEx305[str-1p::odr-1; elt-2p::gfp] | Ferkey lab |
| FG559 | odr-1(n1936); udEx306[str-1p::odr-1; elt-2p::gfp] | Ferkey lab |
Acknowledgments
We thank R. Morimoto for generous hospitality; C. Bargmann, D. Ferkey, and R. Pocock, for sharing strains; and F. Schroeder for ascarosides. We derived some information from Wormbase; it is supported by grant U41 HG002223 from the National Human Genome Research Institute at the NIH, the UK Medical Research Council, and the UK Biotechnology and Biological Sciences Research Council. Several C. elegans strains were obtained from the Caenorhabditis Genetics Center (CGC) (Minneapolis, MN, USA) which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
Funding
This work was funded in part by the NIH (R01GM126125) grant to I.R.
Reviewed By
Douglas PortmanHistory
Received: December 9, 2021Revision received: January 7, 2022
Accepted: January 11, 2022
Published: January 13, 2022
Copyright
© 2022 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Aprison, EZ; Ruvinsky, I (2022). ODR-1 acts in AWB neurons to determine the sexual identity of C. elegans pheromone blends. microPublication Biology. 10.17912/micropub.biology.000507.Download: RIS BibTeX