Abstract
Cellular function is regulated by the concentration of intracellular and extracellular ions, including pH. Ion channels and transporters that mediate the flux/transport of protons and bicarbonate (HCO3–) are the chief regulators of pH. In the nervous system, due to their high electrical activity, neurons tend to produce and excrete large amounts of acids. On the contrary, glial cells have been proposed to be key contributors of pH buffering. We published that the Cl–/HCO3– permeable channel CLH-1 mediates intracellular pH buffering of C. elegans Amphid sheath (AMsh) glia at baseline. We show here that, under physiological conditions, clh-1 knock out worms show reduced HCO3– extrusion from AMsh glia, suggesting that CLH-1 may help prevent cellular alkalinization. This function becomes even more apparent when animals are grown on plates enriched with HCO3–. We speculate that CLH-1 might function as a regulator of extracellular pH.
Description
Cellular function is regulated by both intracellular and extracellular pH. Proton and bicarbonate transporters and exchangers, as well as ion channels permeable to protons and bicarbonate (HCO3–) participate to pH regulation (Chesler, 2003). Importantly, channels and transporters that regulate extracellular pH have the potential of influencing the function of nearby cells, making them particularly critical for the function of a tissue or organ as a whole. Extracellular pH regulation, and in particular buffering of extracellular acid loads, is especially important in the nervous system where high neuronal activity leads to the production and release in the extracellular space of a significant amount of acid.
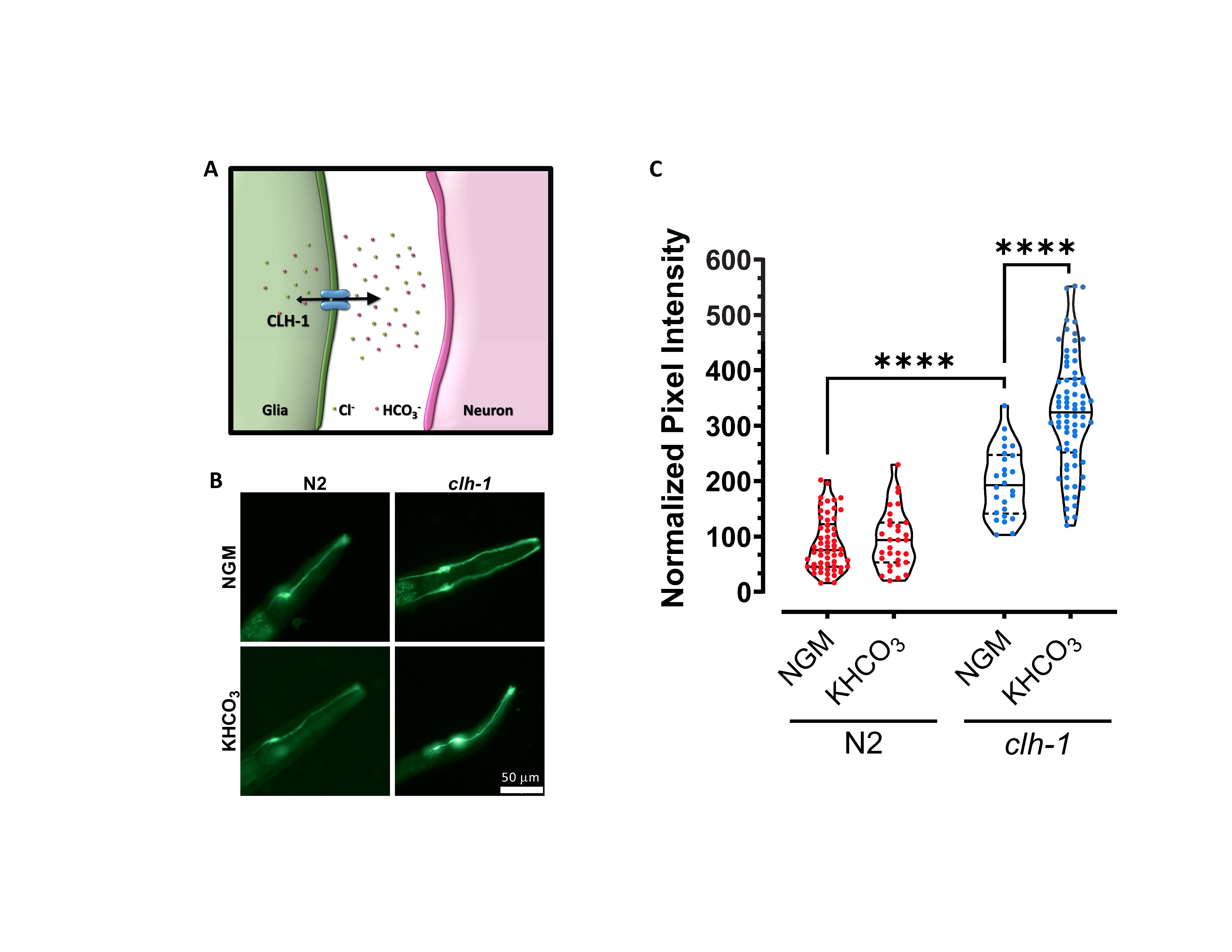
We published that the ClC Cl– channel CLH-1 is expressed in the Amphid sheath glial cells (AMsh glia) of the C. elegans amphid apparatus (Grant et al., 2015). These glial cells enwrap the sensory dendrites of 12 pairs of sensory neurons and are important for the structure and the function of these neurons (Bacaj et al., 2008, Singhvi et al., 2016, Raiders et al., 2021, Wang et al., 2008, Wang et al., 2012, Fernandez-Abascal et al., 2021, Wallace et al., 2016, Oikonomou et al., 2011, Razzauti and Laurent, 2021). Using the genetically encoded pH indicator Superecliptic pHlourin, we showed that CLH-1 is needed for influx of HCO3– into the AMsh glia at baseline pH, following removal of HCO3– from the extracellular space (Grant et al., 2015). However, by electrophysiological analysis of CLH-1 ionic currents in Xenopus oocytes and mammalian HEK cells, we also showed that this channel conducts ions preferably from the inside to the outside of the cell (Grant et al., 2015). This finding suggests that CLH-1 might mediate HCO3– efflux in vivo thereby resulting in regulation of the pH outside of AMsh glia, potentially even in the microenvironment between AMsh glia and sensory dendrites (Fig. 1A). Consistent with CLH-1-mediated efflux of ions, we found that CLH-1 mediates Cl– efflux from AMsh glia needed for GABA regulation of ASH neurons’ excitability upon nose touch stimulation (Fernandez-Abascal et al., 2021).
To determine whether CLH-1 mediates HCO3– efflux in vivo, we compared pHlourin fluorescence in AMsh glia of wild type and clh-1 knockout mutants grown on control plates and on plates supplemented with 150 mM KHCO3 (Johnson et al., 2020, Singhvi et al., 2016, Fernandez-Abascal et al., 2021) (Fig. 1B-C). pHlourin fluorescence intensity is proportional to the alkalinity of the cell, thus, higher fluorescence indicates that the intracellular environment is at a higher (more alkaline) pH. First, we found that AMsh glia of clh-1 knockout animals grown on control plates had on average a fluorescence intensity twice as high as in wild type (Fig. 1B-C). This result suggests that efflux of HCO3– from the AMsh glia is reduced in clh-1 knockout as compared to wild type. Second, AMsh glia pHlourin fluorescence of wild type animals grown on HCO3– enriched plates was like the fluorescence intensity seen in animals grown on control plates (Fig. 1B-C). This result indicates that in wild type AMsh glia an efficient HCO3– extrusion mechanism keeps the pH relatively stable despite the challenge with high concentration of HCO3–. On the contrary though, the AMsh glia of clh-1 knockout animals grown on HCO3– enriched plates had a fluorescence intensity that on average was one and half times higher than the fluorescence intensity of these cells in animals grown on control plates (Fig. 1B-C). This result confirms that HCO3– extrusion from AMsh glia is severely impaired in clh-1 knockout. These data also suggest that when challenged with 150 mM HCO3– AMsh transport HCO3– inside the cell in a CLH-1-independent manner. A CLH-1-independent HCO3– transport into AMsh glia was previously suggested by pH imaging of these cells under acid load challenge (Grant et al., 2015).
How do these results reconcile with our previous report (Grant et al., 2015)? The directionality of HCO3– flux through a channel depends on the driving force for the ion and the membrane potential. In our previous work that showed CLH-1-mediated HCO3– influx at baseline, pH imaging experiments were conducted on AMsh bathed in Hepes buffer and subsequently perfused with 20 mM HCO3–. Under those conditions, HCO3– was initially depleted from the intracellular milieu, resulting in an electrochemical gradient for HCO3– favoring inward current at negative resting membrane potential which was supported by our results. In the experiments reported here, growth in NGM control plates is expected to result in a physiological concentration of HCO3– of ~20 mM both inside and outside the AMsh cells (Harpur, 1974). Thus, at a negative resting membrane potential, HCO3– current is expected to be outward, which is supported by our results showing that clh-1 knockout leads to accumulation of HCO3– in the cell (Fig. 1C). When worms are grown in high HCO3– the reversal potential for this ion will tend to become more negative but it seems to remain more positive than the resting membrane potential, thus, continuing to favor HCO3– efflux through CLH-1. If clh-1 is knocked out, then efflux is impaired and accumulation of HCO3– is favored. Interestingly, we noticed that growth on high K+ (deriving from the dissociation of KHCO3) does not seem to dramatically change the resting potential of the cell which appears to remain still more negative than the predicted reversal potential for HCO3– under these conditions (-47 mV). We note though that the exact concentration of K+ or HCO3– in worms grown on KHCO3 plates is not known and may be different between the two ions.
To conclude, we propose that CLH-1 might regulate the pH outside AMsh glia, in particular in the microenvironment around the dendrites of the amphid sensory neurons, and that this might result in regulation of neuronal output. This CLH-1-dependent mechanism of regulation of neuronal function might be in addition to providing Cl– ions for GABA signaling in nose touch avoidance (Fernandez-Abascal et al., 2021). Future imaging and behavioral experiments using an extracellular pH sensor and CLH-1 mutants with altered Cl– and HCO3– permeability will test this hypothesis.
Methods
Request a detailed protocolC. elegans growth and maintenance: Animals were grown at 20°C on standard nematode medium (NGM) seeded with Escherichia coli (strain OP50). Experiments were performed on young adult hermaphrodites.
Plate supplementation: NGM plates were prepared as usual and a solution containing autoclaved KHCO3 was added to the media right before pouring into plates, to a final concentration of 150 mM. Worms were grown in supplemented plates from egg to young adults.
Worm bleaching: Gravid adults were collected from plates using M9 buffer and transferred into tubes for centrifugation (5 mins at 4300 rpm). Next, the pelleted worms were treated with a solution containing 0.1 M NaOH and 22.7% commercial bleach for 5-10 minutes. When about 90% of the eggs were released, they were washed with M9 twice. Eggs were then seeded onto control and supplemented plates.
C. elegans strains: The following strains from (Grant et al., 2015) were used: BLC44 Ex405[pT02B11.3::pGM87] and BLC295 clh-1(ok658); Ex405[pT02B11.3::pGM87]. The BLC295 strain was obtained by crossing clh-1(ok658) with BLC44. The clh-1(ok658) strain used in the cross described was RB833.
Fluorescence microscopy: Animals were immobilized with 100 mM NaN3 on 2% agarose pads. An Evos FL Auto 2 Imaging System (Invitrogen) equipped with a 40x objective (Olympus) was used to acquire the images of the AMsh cell body. An average of 6 stacks (0.6 µm each) on the Z axis were acquired per cell using the Evos FL Auto 2 software. Stacks were then processed using the “Z project” plugin (maximum intensity as projection type) from Fiji (ImageJ software). The resulting images were used to quantify the pixel intensity of the AMsh glia as an averaged intensity per area. Data were normalized to N2 in NGM conditions.
Acknowledgments
We thank Robert W. Keane for sharing equipment essential to data collection.
References
Funding
This work was supported by NIH Grants R01s NS070969 and NS105616A1.
Reviewed By
Hirofumi Kunitomo and AnonymousHistory
Received: December 2, 2021Revision received: December 29, 2021
Accepted: January 3, 2022
Published: January 12, 2022
Copyright
© 2022 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Fernandez-Abascal, J; Bianchi, L (2022). The ClC Cl– channel CLH-1 mediates HCO3– efflux from the amphid sheath glia in C. elegans. microPublication Biology. 10.17912/micropub.biology.000510.Download: RIS BibTeX