Abstract
METTL3, the enzyme that catalyzes the m6A RNA modification in Drosophila is highly conserved and essential in various eukaryotic organisms. Mettl3 and its homologs have been linked to biological processes such as gametogenesis. We focused on characterizing the role of METTL3 in Drosophila spermatogenesis. We used the Gal4-UAS system to ubiquitously knockdown Mettl3 in both somatic cyst cells and germline cells. Using immunostaining and confocal microscopy, we found spermatid bundles mislocalize in testes that contain the morphologically abnormal swollen apical tip. Our result suggests Mettl3 knockdown using TRiP.GL01126 results in spermatogenesis aberrations.
Description
METTL3 contains the catalytic subunit of the m6A methyltransferase complex. METTL3 serves as a “writer” protein during mRNA processing as it methylates nitrogen at the sixth position of adenosine residues in eukaryotic organisms (Rottman et al., 1994). Mettl3 and its homologs have been shown to be crucial for developmental events in eukaryotic organisms (Hsu et al., 2017). Developmental processes such as gametogenesis depend on the proper functioning of METTL3. Previous research in Drosophila spermatogenesis suggests that somatic cyst cell specific knockdown of Mettl3 leads to misregulation of an essential protein profilin (chic), which disrupts the somatic permeability barrier (Rockwell and Hongay, 2020). Using spermatogenesis as a model system, our research uses ubiquitous knockdowns to further elucidate the role of METTL3 in spermatogenesis.
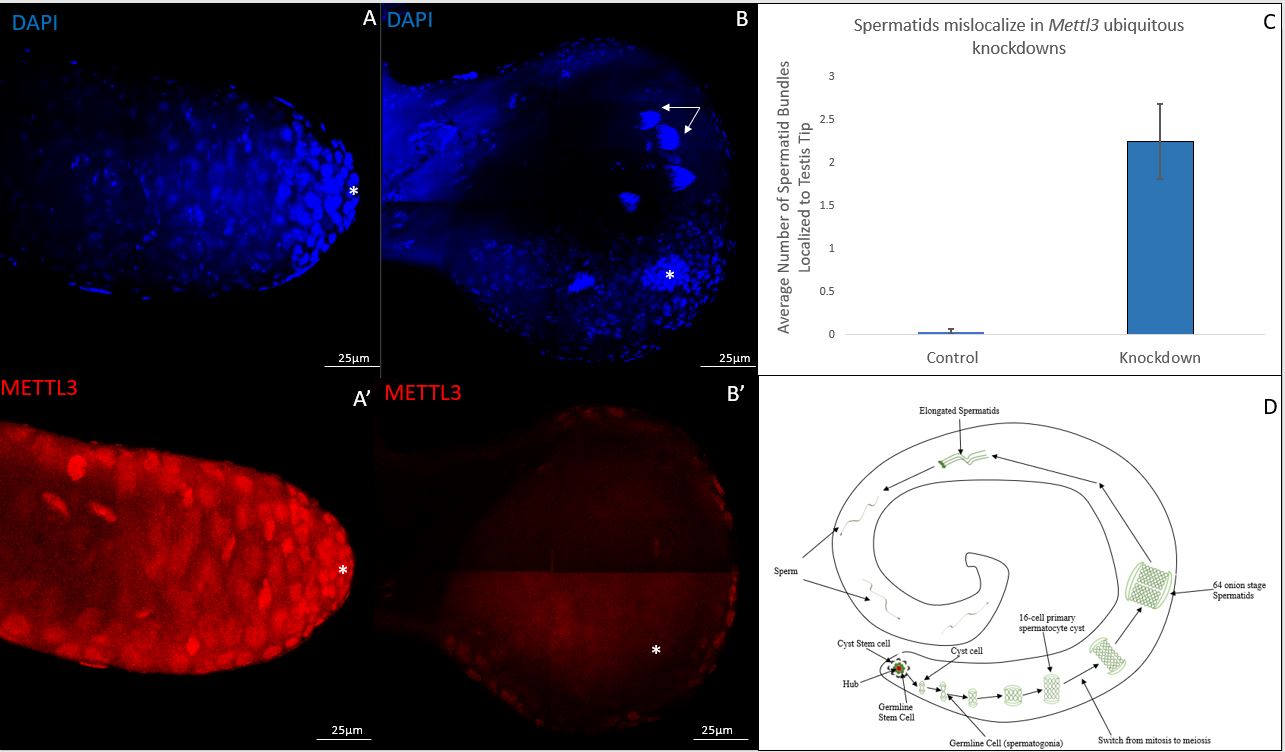
Spermatogenesis is an ideal system for characterizing cell localization patterns. In our investigation, we aimed to determine if readily identifiable localization patterns were disrupted in METTL3 deficient testes. To elucidate the role of METTL3 related to our investigation aim, we performed a ubiquitous knockdown to reduce METTL3 in soma and germline cells. We utilized an RU-486 inducible Actin 5C driver system to ubiquitously knockdown Mettl3. We dissected testes from balanced controls and ubiquitous knockdowns. We found that 48.8% of knockdown testes had the previously reported swollen tip phenotype (Rockwell and Hongay, 2020). Additionally, we found that the swollen tip phenotype corresponded to an increase in spermatid bundles localizing to the apical tip of the testis (Figure 1A, 1B and 1C) (Wu et al., 2016). In approximately half of the testes with a swollen tip, the hub appeared to move inward from its normal position at the apical tip. We did not see a significant difference in morphologically normal knockdown testes and controls. For all testes, whole-mount antibody staining was done to confirm METTL3 depletion (Figure 1A’ and 1B’).
Germline localization patterns in spermatogenesis have been well characterized. For example, spermatogonia are normally found at the apical tip of the testis while spermatid bundles are normally localized close to tail of the testes (Demarco et al., 2014) (Figure 1D). Our work suggest spermatid bundles in ubiquitous Mettl3 knockdowns are improperly localizing from their traditional population in the tail to the tip of the testis. The swollen tip phenotype seen in almost half of knockdowns suggests that Mettl3 potentially plays a critical role in maintaining normal testis morphology as well. Cell mislocalization and tissue expansion phenotypes are associated with numerous developmental conditions. It is important to note that our work involved using a single RNAi construct. Future work using additional RNAi constructs or mutants needs to be conducted to ensure that the phenotypes observed are directly correlated to METTL3 deficiency. If direct correlation is confirmed, additional work will be needed to elucidate the mechanisms responsible for the observed phenotypes in METTL3 deficient backgrounds. Understanding the role of METTL3 will help us gain a greater understanding of the m6A epitranscriptomic modification and subsequently biological processes associated with the modification.
Methods
Request a detailed protocolDrosophila stocks were fed as previously reported (Rockwell and Hongay, 2020).
Crossing: Mettl3 (FBgn0039139) was ubiquitously knocked down by crossing BL9431 P{ry[+t7.2]=hsFLP}12, y[1] w[*]; P{w[+mC]=UAS-GFP.S65T}Myo31DF[T2]; P{w[+mC]=Act5C(-FRT)GAL4.Switch.PR}3/TM6B, Tb[1] with responder line BL41590 y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.GL01126}attP2/TM3, Sb[1] . Progeny were induced with RU-496 progesterone analog at the L3 stage of development. Inducement prior to L3 resulted in premature death in all progeny containing driver and responder. Progeny were sorted based on tubby and stubble phenotypes.
Immunostaining: Young 1-to-3-day old male progeny testes were fixed and permeabilized as previously described (Bonaccorsi and Gatti, 2017; Bonaccorsi et al., 2011, 2012). Testes were stained with DAPI as previously described (Rockwell and Hongay, 2020).
Confocal imagery: Testes images were obtained from prepared slides using the Nikon-700 series confocal microscope.
Reagents
| Strain | Genotype | Source |
| BL9431 | P{ry[+t7.2]=hsFLP}12, y[1] w[*]; P{w[+mC]=UAS-GFP.S65T}Myo31DF[T2]; P{w[+mC]=Act5C(-FRT)GAL4.Switch.PR}3/TM6B, Tb[1] | Bloomington Drosophila Stock Center |
| BL41590 | y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.GL01126}attP2/TM3, Sb[1] | Bloomington Drosophila Stock Center |
References
Funding
This work was supported by the Susquehanna University Summer Partners Research Grant to LR and AR.
Reviewed By
AnonymousHistory
Received: August 31, 2021Revision received: January 2, 2022
Accepted: January 7, 2022
Published: January 18, 2022
Copyright
© 2022 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Rowe, L; Rockwell, AL (2022). Ubiquitous Knockdown of Mettl3 using TRiP.GL01126 Results in Spermatid Mislocalization During Drosophila Spermatogenesis. microPublication Biology. 10.17912/micropub.biology.000511.Download: RIS BibTeX