Abstract
Herein, we tested the ability of UNC-33L to rescue dauer formation, lifespan, and locomotion defects of unc-33(mn407) mutants. Results show that the presence of UNC-33L does not rescue the defective dauer phenotype in unc-33(mn407) mutants. However, UNC-33L significantly rescued premature death and uncoordinated locomotion in young unc-33(mn407) adults. The degree of UNC-33L-mediated rescue was less noticeable as the nematodes aged, denoting that both age and the presence of UNC-33L interact in the production of the phenotypes.
Description
C. elegans have a short life cycle that consists of a larva molting into a reproducing adult by passing through 4 larval stages (L1, L2, L3, and L4). Under favorable conditions, the nematode will go through all 4 larval stages (Wolkow and Hall 2015). On the other hand, if conditions become unfavorable, the nematode will shift into dauer formation right after the L2 phase (L2d). During this alternative life cycle, C. elegans become stress-resistant, developmentally arrested, and long-lived (Albert and Riddle 1983). In addition, they undergo morphological remodeling that allows them to adapt to harsh conditions (Cassandra and Russell 1975). Dauers develop a thickened cuticle, undergo body constriction, and cell compaction. The thickened cuticle inhibits environmental toxins, such as detergent from affecting internal organs (Wolkow and Hall 2015). Once conditions become favorable, the nematode will exit out of the dauer phase and continue on to become a reproducing adult.
Although wild-type (WT) nematodes can enter this dauer phase, unc-33 mutants are unable to enter this alternative life cycle, and the reasoning behind this is unclear. The C. elegans UNC-33 protein is the ortholog of human microtubule-binding protein CRMP2 (Tsuboi et al. 2005). UNC-33/CRMP2 plays a role in microtubule organization and axon-dendrite sorting to establish an organism’s nervous system (Maniar et al. 2011). In the absence of UNC-33 polypeptides, nematodes present with uncoordinated locomotion and defects in axonal elongation (Li et al. 1992). In humans, studies have found CRMP2 defects in patients with Alzheimer’s and other age-related neurodegenerative diseases (Ban et al. 2013; Arey and Murphy, 2017).
Aging is a biological phenomenon that occurs in all life forms due to the decline and deterioration of cells and organs. Aging cells and organs progressively accumulate dysfunctional cellular components, are subject to oxidative damage, and have a decline in protein turnover rates and housekeeping mechanisms (Kurz et al. 2007; Rajawat and Bossis 2008). Evidence collected from the study of aging suggests that the continuous removal of dysfunctional organelles and the synthesis of new ones allow for a delay in the aging process, gleaning that there are mechanisms extending longevity and inhibiting aging. Further exploring the aging mechanisms is the objective of this study.
To analyze the effects of the unc-33 gene in C. elegans further, we investigated the role of UNC-33L in dauer development, lifespan, and age-dependent locomotion in unc-33(mn407) mutants. UNC-33 codes for three alternatively spliced isoforms UNC-33L (Large), UNC-33M (Medium), and UNC-33S (Small) with each isoform varying at the N terminus (Maniar et al. 2011). unc-33(mn407) mutants contain a 500 bp deletion in the unc-33 ORF and produce none of the alternatively spliced isoforms of UNC-33 (Tsuboi et al. 2005 and Maniar et al. 2011). In previous studies, Maniar and colleagues found that UNC-33L was able to fully rescue uncoordinated locomotion, egg-laying defects, and mislocalization of RAB-3::mCherry and SAD-1::GFP in unc-33 (mn407) mutants, suggesting that UNC-33L is sufficient to restore neuronal activity to WT levels in the unc-33 mutant background (Maniar et al. 2011). To this end, we hypothesize that the introduction of UNC-33L in an unc-33 null mutant will result in a rescue of the dauer defective phenotype, lifespan, and age-dependent locomotion.
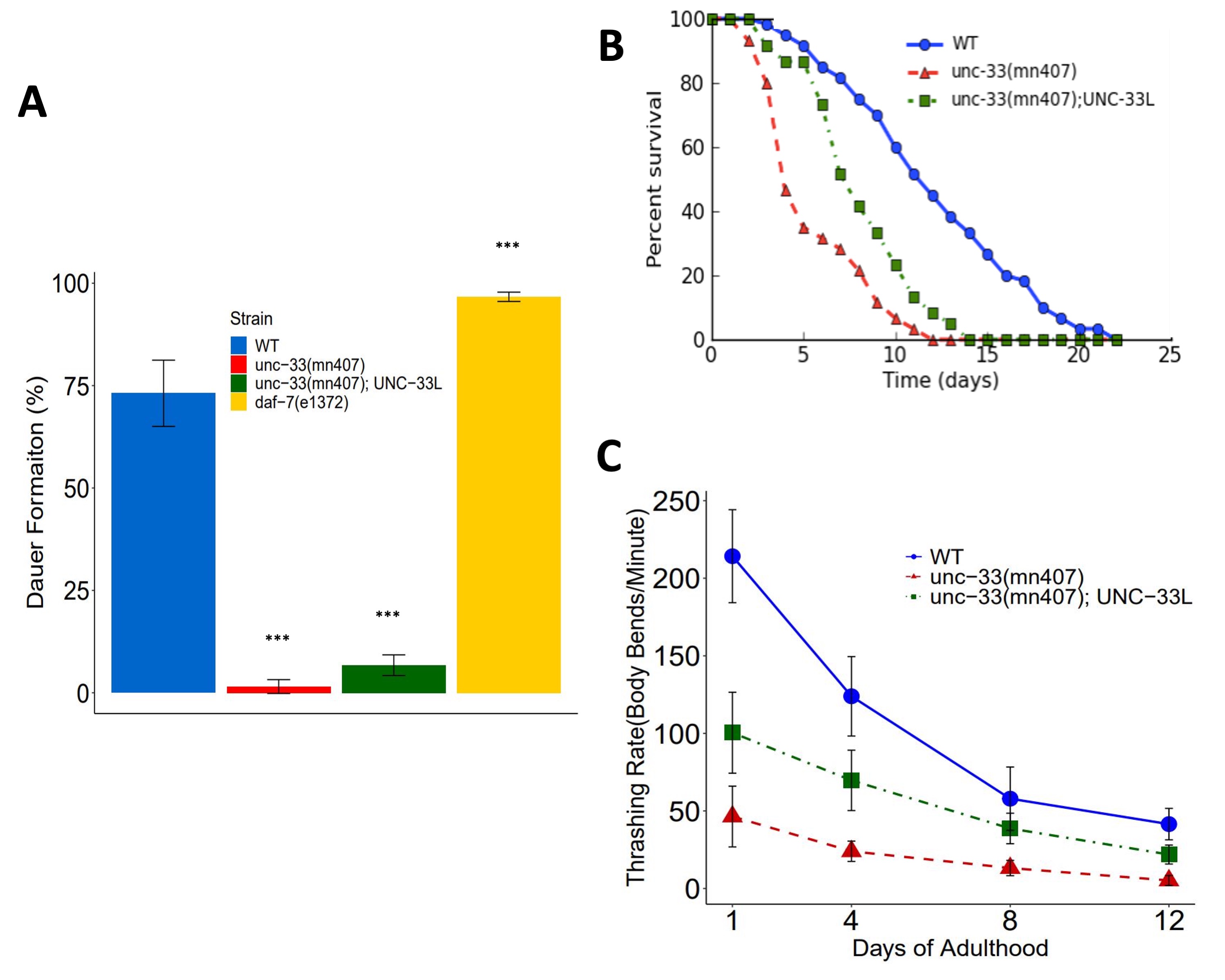
To test the rescue of the dauer defective phenotype, we treated nematodes with 1% SDS and assessed dauer formation. Analysis of our results show that unc-33(mn407);UNC-33L transgenic nematodes were unable to produce dauers (6.74% +/- 0.025%), a phenotype characteristic of unc-33(mn407) mutants (3.23% +/- 0.026%). Meanwhile, dauer formation was scored as 96.68% +/- 0.011% in daf-7(e1372) mutants and 73.14% +/- 0.081% in WT nematodes (Figure 1A). Examinations of pairwise comparisons via the Tukey post-hoc resulted in no statistically significantly different results between unc-33(mn407); UNC-33L transgenics and unc-33(mn407) mutants (p-value= 4.98E-01). However, pairwise comparisons between the remaining strains resulted in statistically significantly different results as follows: unc-33(mn407); UNC-33L vs WT p-value ≦0.0001; daf-7(e1372) vs unc-33(mn407) p-value ≦0.0001; unc-33(mn407); UNC-33L vs daf-7(e1372) p-value ≦0.0001, WT vs unc-33(mn407) p-value ≦0.0001; and WT vs daf-7(e1372) p-value ≦0.0001. These results suggest that the addition of UNC-33L was not sufficient in rescuing the defect in dauer formation of the unc-33(mn407) mutants as scored by the 1% SDS protocol.
To test the effects of UNC-33L rescue in lifespan, we quantified the longevity in unc-33 mutants and found a partial rescue denoted by a p-value ≦0.0001 in the pairwise comparison of unc-33(mn407) vs unc-33(mn407); UNC-33L transgenics using a Kaplan-Meier test. Analysis of WT vs unc-33(mn407); UNC-33L pairwise comparison had a p-value ≦0.0001. The Kaplan-Meier estimates of 50 percent mortality were 12 days of adulthood for WT, 8 days of adulthood for unc-33(mn407); UNC-33L transgenics, and 5 days of adulthood for unc-33(mn407) mutants. Additionally, examinations of lifespan assays showed that animals expressing UNC-33L had death rates similar to WT during days 1-5 of adulthood. Following day 5 of adulthood, unc-33(mn407); UNC-33L nematodes showed an increase in death rates, resulting in a phenotype intermediate between WT animals and unc-33(mn407) mutants (Figure 1B).
Lastly, in testing whether locomotion defects would be rescued in the presence of UNC-33L, we quantified thrashing rates in unc-33 mutants and found a significant difference in thrashing rates between unc-33(mn407) vs unc-33(mn407); UNC-33L transgenics (Figure 1C). Analysis of the data using two-way ANOVA showed an interaction effect between strains and age, demonstrating that the degree of the rescue of thrashing is dependent on the age of the nematodes (Figure 1C).
Together, lifespan and locomotion studies suggest that expression of UNC-33L alone has a greater effect on survivability and motility earlier in adulthood. However, this positive effect declines as the animals’ age, resulting in the partial rescue of lifespan and locomotion defects.
Methods
Request a detailed protocolSynchronization of nematodes:
To synchronize nematodes, adult worms from each strain were floated in DI water, transferred to 15 mL conical tubes, and centrifuged for 1 minute at 3757 x g at 20 ºC. After centrifugation, the supernatant was discarded, and 7 mL of alkaline bleach plus 7 mL of DI water were added to the pellet. The tube containing the pelleted worms and diluted alkaline bleach was incubated for 7 minutes on a rocker at room temperature. After incubation, the worm suspension was centrifuged for 5 minutes at 3757 x g at 20 ºC, and the supernatant was discarded. Next, the pellets were washed using 10 mL of M9 buffer and centrifuged for 1 minute at 9221 x g at 20ºC after each wash. A total of three washes were performed. After the final centrifugation, the supernatant was discarded, and the pellets containing eggs were pipetted onto seeded plates.
Lifespan Assay:
The longevity of nematodes was quantified by examining 20 synchronized young adults per strain and assessing their survival every 24 hours until death. Synchronized populations of worms were cultivated on NGM agar plates containing OP50 E. coli bacteria at 20 ºC. Once they’ve reached adulthood, 20 nematodes per strain were transferred onto a new plate and recorded as day 1 of adulthood. The survival of nematodes was monitored daily by transferring living animals to a new seeded plate with OP50 E. coli until all remaining animals died. Survival was recorded as the percent ratio of living to dead nematodes.
Locomotion Assay:
The locomotion of the nematodes was quantified by examining 20 adults per strain and assessing their body thrashes in liquid at various ages of their adult life (1, 4, 8, and 12 days of adulthood). Synchronized populations of worms were cultivated on NGM agar plates containing OP50 E. coli bacteria at 20 ºC. Once they’ve reached the specified adult age, individual nematodes were picked and transferred to wells containing 1 mL of DI water. Once transferred, each nematode was allowed to acclimate for 10-15 seconds before evaluating thrashing rates. Locomotion is quantified by counting the thrashes in 30 seconds, and the thrashing rate was expressed as body bends per minute.
SDS Protocol:
To assess dauer formation, we examined 200 starved worms per strain, per trial. Animals were starved for 5 days at 20 ºC, with the exception of daf-7(e1372) which were kept at 25 ºC for 5 days. After the 5-day starvation period was reached, animals were resuspended with 1 mL DI water and transferred into 1.5 mL tubes. Worms were centrifuged for 1 minute at 9221 x g and pellet worms were treated with 1 mL of 1% SDS for 20 min at room temperature on an orbital shaker. After the 20 minute incubation, worms were centrifuged for 1 minute at 9221 x g and the supernatant was removed. Pelleted worms were washed 3 times using 1 mL of M9 buffer and centrifuged for 1 minute at 9221 x g at 20ºC after each wash. After the last wash, 100uL of pellet worms were transferred onto an NGM plate with no bacteria and the percent survival was quantified. Alive animals were picked one by one and then burned to ensure adequate scoring.
Statistical Analysis:
All data analysis was performed using R. For dauer formation, hypothesis testing was evaluated using one-way ANOVA after determining normality via the Shapiro-Wilks test and homogeneity of variance via the Levene test. A Post-Hoc Tukey’s test was used for pairwise comparisons. Lifespan assays were analyzed via Kaplan-Meier Survival Analysis using Oasis programming (Yang et al. 2011). The locomotion data was interpreted using two-way ANOVA after determining normality via the Shapiro-Wilks test and homogeneity of variance via the Levene test.
Reagents
N2 (WT), SP1382 unc-33(mn407), and CB1372 daf-7(e1372) nematodes were obtained from the Caenorhabditis Genetics Center.
Strain CX13244 kyIs445 [PVD::mcherry::RAB-3 + PVD::SAD-1::GFP]; unc-33(mn407); kyEx3861 [tag-168::UNC-33L (20 ng/μl), unc-122::DsRed (20 ng/μl)], was obtained from Cori Bargmann’s Lab (Maniar et al.. 2011). Strain CX13244 was created by microinjecting a plasmid driving the expression of an UNC-33L cDNA under the regulation of the pan-neuronal tag-168 promoter.
Acknowledgments
The CX13244 strain was provided by Cori Bargmann at the Rockefeller University and all other strains were provided by the CGC, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). Alexia Samaro and Dr. Charles Hauser were instrumental in helping with the statistical analysis.
References
Funding
Funding for this work has been provided by the National Science Foundation Awards # 1748523, 1826871,182282, the Ronald E. McNair Postbaccalaureate Achievement Program, and the Department of Biological Sciences at St. Edward’s University.
Reviewed By
AnonymousHistory
Received: December 13, 2021Revision received: January 12, 2022
Accepted: January 15, 2022
Published: January 24, 2022
Copyright
© 2022 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Lopez, ME; Vacio, AM; Cantu, J; Holgado, A (2022). UNC-33L partially rescues life span and locomotion defects in unc-33 mutants but fails to rescue dauer formation defects.. microPublication Biology. 10.17912/micropub.biology.000515.Download: RIS BibTeX