Abstract
Animals broadcast small molecule pheromones that can alter behavior and physiology in conspecifics. Neuronal circuits that regulate these processes remain largely unknown. In C. elegans, male-enriched ascaroside sex pheromone ascr#10, in addition to behavioral effects, expands the population of germline precursor cells in hermaphrodites. Previously, we identified several sensory neurons required for this effect. We also found that feedback from egg laying acts via serotonergic signaling to license the pheromone response in reproducing adults. Here, using newly available reagents, we confirm and extend several of our previous conclusions: a) the ADL neurons are essential for the ascr#10 response, b) phasmid neurons (PHA and PHB) are unlikely to be involved in the ascr#10 response, c) the mod-1 receptor is the main conduit of the serotonergic feedback from egg laying, and d) serotonin remains the only currently known signal of this feedback. Our findings better define the neuronal circuits that mediate the germline response to the major male pheromone.
Description
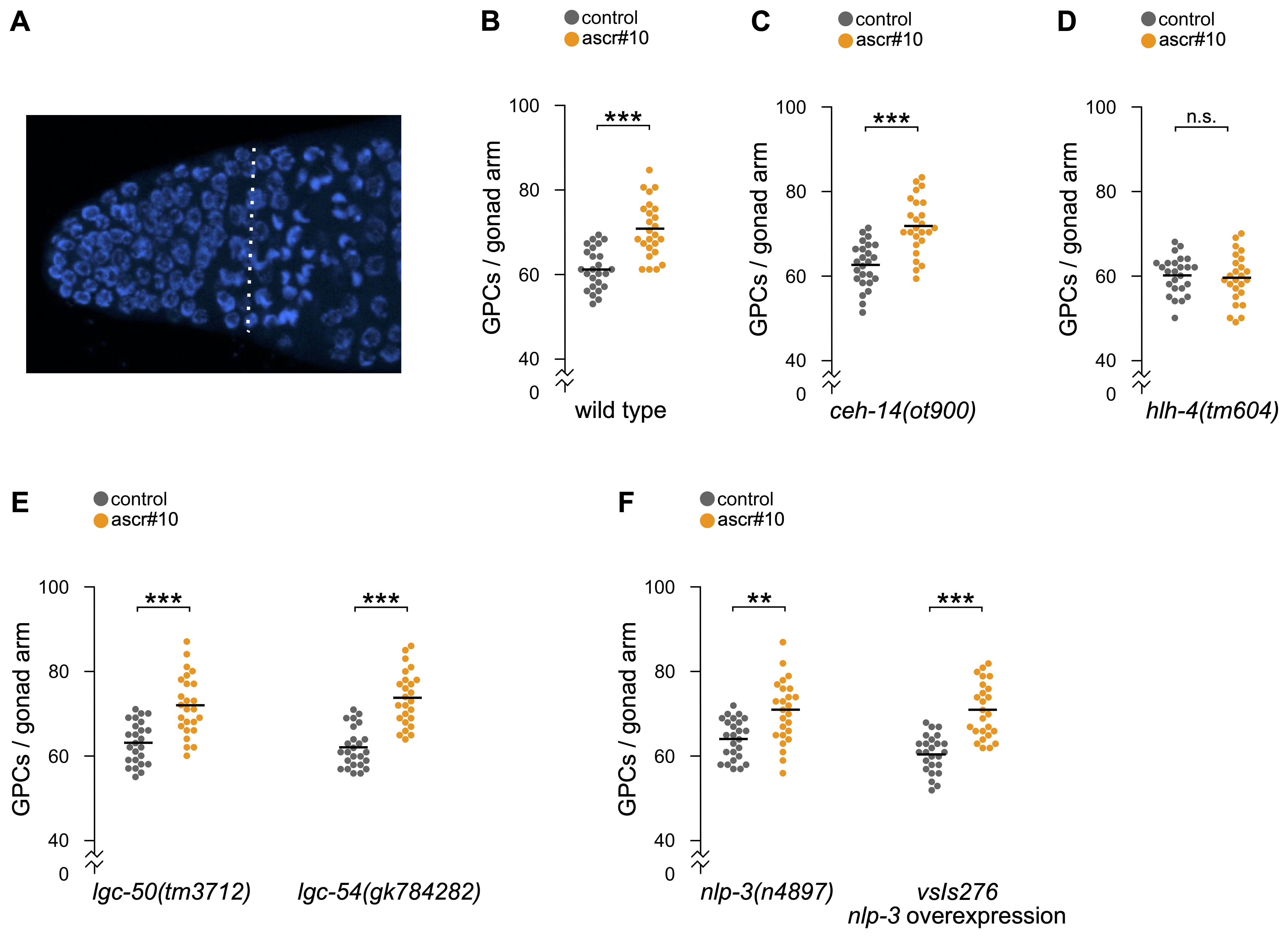
The distal-most portion of the hermaphrodite germline in C. elegans is referred to as the Progenitor Zone (Figure 1A). Most cells in this population are mitotically-cycling germline precursors, but some have progressed as far as the early stages of prophase of meiosis I (Fox et al., 2011). The size of the Progenitor Zone is sensitive to a variety of environmental factors (Hubbard and Schedl, 2019). For example, in the presence of physiological concentrations of ascr#10, the most abundant male-enriched excreted ascaroside (Izrayelit et al., 2012), hermaphrodites have an enlarged population of germline precursor cells (Figure 1B; (Aprison and Ruvinsky, 2016)).
Previously, we identified several neurons involved in the germline response to ascr#10. Because loss-of-function mutations in ocr-2 and osm-9 genes eliminated the germline response, we focused on six pairs of sensory neurons – amphids ADF, ADL, ASH, and AWA and phasmids PHA and PHB – in which expression of these two genes overlaps (Aprison and Ruvinsky, 2017). A recent single cell RNAseq atlas supports the idea of a narrow overlap between expression patterns of ocr-2 and osm-9, identifying PVD as the only additional neuronal type in the overlap (Taylor et al., 2021). In the original study, we focused on amphid neurons because loss of a LIM homeobox gene ceh-14 did not appear to alter the ascr#10 response (Aprison and Ruvinsky, 2017). The ceh-14 gene is thought to be expressed in ~10 (Kagoshima et al., 2013) to ~20 (Taylor et al., 2021) neuronal types, including phasmid sensory neurons PHA and PHB. It was not clear whether the ceh-14(ch3) allele tested in our original study (Aprison and Ruvinsky, 2017), caused a complete and stable loss of function. More recently, a new mutant allele ceh-14(ot900), a near complete deletion of the locus, became available (Bayer and Hobert, 2018). Hermaphrodites carrying this allele had the same germline response to ascr#10 as the wild type animals (Figure 1C), confirming our original conclusion that PHA and PHB neurons are dispensable for the germline response to ascr#10.
Of the four pairs of amphid neurons implicated by the ocr-2/osm-9 overlap (ADF, ADL, ASH, and AWA), our original study focused on ADL because expression of the wild type OCR-2 protein in these, but not in the other three pairs, rescued the ocr-2(lf) inability to respond to ascr#10 (Aprison and Ruvinsky, 2017). To obtain additional evidence for the role of ADL neurons, we tested a loss-of-function mutation in the HLH-4 transcription factor that defines ADL identity (Masoudi et al., 2018). This gene appears to be expressed exclusively in ADL neurons, as judged by a fosmid-based reporter transgene (Masoudi et al., 2018) and the single cell RNAseq atlas (Taylor et al., 2021). hlh-4(tm604) animals showed no increase of germline precursor cells in the presence of ascr#10 (Figure 1D), confirming our original conclusion that ADL neurons are required for the germline response to this male pheromone.
The ability of ascr#10 to enlarge the population of germline precursor cells is restricted to actively reproducing hermaphrodites because egg laying licenses pheromone response (Aprison and Ruvinsky, 2019b). The feedback of active reproduction involves the serotonin signal from the command motoneurons of egg laying, HSN (Aprison and Ruvinsky, 2019b). Previously, we tested mutants in six genes thought to act as serotonin receptors – ser-1, ser-4, ser-5, ser-7, mod-1, and lgc-40 – and found that only one, the inhibitory serotonin-gated chloride channel mod-1, is essential for the increase of the number of germline precursors on ascr#10 (Aprison and Ruvinsky, 2019a). Since then, two additional serotonin receptors have been reported: lgc-50 that appears to be exclusive for serotonin and lgc-54 that also responds to dopamine and tyramine (Morud et al., 2021). Hermaphrodites carrying mutations in either of these two genes did not discernibly differ from their wild type counterparts (Figure 1E), further supporting the idea that mod-1 is the only currently annotated serotonin receptor required for the germline effects of ascr#10.
Command motoneurons HSN release serotonin along with neuropeptide NLP-3 to stimulate egg laying episodes (Brewer et al., 2019). Because feedback from egg laying, mediated at least in part by serotonin, is required for increasing the number of germline precursors in the presence of ascr#10 (Aprison and Ruvinsky, 2019b), we tested whether NLP-3 plays a role in this feedback. We found that neither nlp-3 loss (nlp-3(n4897)), nor overexpression (nlp-3 (vsIs276)), noticeably affected germline response to ascr#10 (Figure 1F). These results leave serotonin as the only currently known mediator of the feedback from the egg-laying apparatus that limits the ascr#10 effects on the germline to actively reproducing animals.
Methods
Request a detailed protocolWe used standard C. elegans methods as previously published (Aprison and Ruvinsky, 2017, 2019a, b). Briefly, worms were synchronized by hypochlorite treatment and subsequent overnight incubation in M9, after which the synchronized L1 larvae were placed (in groups of ~30) on agar plates seeded with E. coli OP50. 48 hours later, when worms were young, but pre-reproductive adults, they were transferred to OP50-seeded plates that were either control or conditioned with synthetic ascr#10 (gift of F. C. Schroeder). This protocol ensured that all experiments were paired with a control. On Day 5 of adulthood, hermaphrodites were stained with DAPI (4′,6-diamidino-2-phenylindole) as described (Aprison and Ruvinsky, 2016) and the germline precursor cells were counted. The boundary between the germline precursor population and the more proximal transition zone is defined by the appearance of crescent-shaped nuclei that have progressed to leptotene/zygotene stages of meiotic prophase (Crittenden et al., 2006; Hansen et al., 2004). Additional protocol details are available upon request.
Reagents
| Strain | Genotype | Obtained from |
| N2 | wild type | CGC |
| OH15422 | ceh-14(ot900) | CGC |
| OH16755 | hlh-4(tm604) | Hobert lab |
| AQ4347 | lgc-50(tm3712) | Morud lab |
| AQ4493 | lgc-54(gk784282) | Morud lab |
| LX2388 | nlp-3(n4897) | Koelle lab |
| LX2518 | vsIs276 (nlp-3 overexpression) | Koelle lab |
Acknowledgments
We thank R. Morimoto for generous hospitality; O. Hobert, J. Morud, and M. Koelle for sharing strains; and F. Schroeder for ascarosides. We derived some information from Wormbase; it is supported by grant U41 HG002223 from the National Human Genome Research Institute at the NIH, the UK Medical Research Council, and the UK Biotechnology and Biological Sciences Research Council. Several C. elegans strains were obtained from the Caenorhabditis Genetics Center (CGC) (Minneapolis, MN, USA) which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
Funding
This work was funded in part by the NIH (R01GM126125) grant to I.R.
Reviewed By
Ariz Mohammad and AnonymousHistory
Received: December 3, 2021Revision received: January 25, 2022
Accepted: January 30, 2022
Published: February 2, 2022
Copyright
© 2022 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Aprison, EZ; Ruvinsky, I (2022). The roles of several sensory neurons and the feedback from egg laying in regulating the germline response to a sex pheromone in C. elegans hermaphrodites. microPublication Biology. 10.17912/micropub.biology.000523.Download: RIS BibTeX