Department of Biology, Johns Hopkins University, Baltimore, MD, 21218.
Description
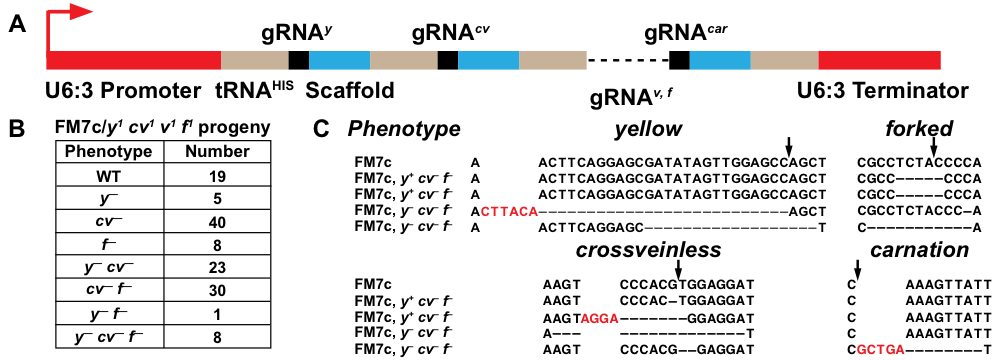
A number of reagents have been generated for CRISPR/Cas9 genome editing in Drosophila (Gratz et al. 2014; Ren et al. 2013; Kondo and Ueda 2013; Port et al. 2014; Port and Bullock 2016). The ability to efficiently express multiple gRNAs from a single vector provides the advantage of creating multiple CRISPR/Cas9 alleles at a time. An elegant design has previously shown that gRNAs separated by tRNAs allow for genomic editing at multiple loci in Drosophila (Port and Bullock 2016). We generated a similar polycistronic gRNA cassette under the control of the Drosophila snRNA:U6:96Ac (U6:3) regulatory sequences. Individual gRNAs were separated by the Drosophila histidine tRNA and targeted the genes yellow (y), crossveinless (cv), vermilion (v), forked (f), and carnation (car) along the X chromosome (A). In theory, after expression of the gRNA cassette, the tRNAs should lead to the formation of secondary RNA structures and be recognized for cleavage by RNase enzymes P and Z (Frendewey et al. 1985; Dubrovsky et al. 2004), releasing the gRNAs for incorporation into Cas9. The gRNA cassette (U6:3-tRNAHIS-gRNAy,cv,v,f,car) was stably integrated into the Drosophila genome and crossed to a Cas9 line under the control of the Act5C promoter (Zhang, Koolhaas, and Schnorrer 2014). Females with the genotype Act5c-Cas9/FM7c; U6:3-tRNAHIS-gRNAy,cv,v,f,car/+ showed y–, cv–, and f– phenotypes while females with the genotype y1 w1/FM7c; U6:3-tRNAHIS-gRNAy,cv,v,f,car/+ were wild type. We then outcrossed Act5c-Cas9/FM7c; U6:3-tRNAHIS-gRNAy,cv,v,f,car/+ females to a y1 cv1 v1 f1 stock and scored germline transmission of editing at y, cv, and f, on the FM7c balancer chromosome. Germline transmission resulted in 14% of progeny not mutant at any locus, 40% were mutant at one locus, 40% were mutant at two loci, and 6% were mutant at three loci (B). We sequenced the targeted genomic sites of two phenotypically y+ cv– f– and two y– cv– f– balancers to compare with the original FM7c chromosome. All flies contained out-of-frame indels at the loci consistent with their phenotypes. None of the flies had mutations at v, and one fly had an in-frame indel at car (C). We are unsure why these flies did not show a car– phenotype, however, our sequencing results indicate that germline transmission of editing events did occur at the car locus. Our data suggests that gRNAs targeting y, cv, f, and car were cleaved from the cassette and loaded into Cas9 allowing for subsequent genomic editing. The ability to express multiple gRNAs from a single vector offers advantages over traditional gRNA vectors for Drosophila and will allow researchers to expand their experimental repertoire for CRISPR/Cas9 genome editing. Practically, polycistronic gRNA vectors can be used to quickly generate and expand new balancer genotypes, that would otherwise be difficult and time-consuming through traditional methods.
Methods
Request a detailed protocolU6:3-tRNAHIS-gRNAy,cv,v,f,car was synthesized by GenScript (Piscataway, NJ) and subcloned into pUASt-attB (Bischof et al. 2007) generating pU6:3-tRNAHIS-gRNAy,cv,v,f,car. pU6:3-tRNAHIS-gRNAy,cv,v,f,car was integrated into the P{CaryP}attP40 landing site (Markstein et al. 2008) through phi-C31 mediated integration (Bischof et al. 2007). Embryo injections for transgenesis and transformant recovery was completed by BestGene (Chino Hills, CA). Sanger sequencing was completed by Genewiz (Plainfield, NJ). Flies were raised on ‘Fly Food B’ (LabExpress, Ann Arbor, MI) at 25°C. A y– phenotype was scored by a reduction in pigmentation of the bristles for y31d on FM7c. All sequences are available upon request.
Reagents
FM7c
FM7c, cvWJB fPDB
y1 v1; P{CaryP}attP40
y1 M{vas-int.Dm}ZH-2A w*
y1 M{Act5C-Cas9.P.RFP-}ZH-2A w1118 DNAlig4169
y1 w1; P{y+t7.7 w+mC=U6:3-tRNAHIS-gRNAy,cv,v,f,car}attP40
References
Funding
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to BO.
Reviewed By
Danny MillerHistory
Received: August 28, 2018Accepted: November 1, 2018
Published: November 13, 2018
Copyright
© 2018 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Benner, L; Oliver, B (2018). Drosophila balancer reengineering using polycistronic gRNA for CRISPR/Cas9 gene editing. microPublication Biology. 10.17912/sg7d-sd61.Download: RIS BibTeX