Description
Reactive oxygen species (ROS) contribute to neuronal degeneration by readily reacting with cellular components, consequently breaking down cellular integrity. Excess ROS often leads to oxidative stress, which results from destabilization of the organism’s ability to control the balance between antioxidants and free radicals (Chandra et al. 2015). The ubiquitin-proteasome system helps to regulate oxidative stress and overall damage to cellular components by forming chains of ubiquitin polypeptides on cellular proteins; these chains then serve as a signal to break down the attached protein (Hershko et al. 1983). Mutation of UBE3B, an E3 ubiquitin ligase, has been found to lead to Blepharophimosis-Ptosis-Intellectual-Disability Syndrome (BPID) in human infants, indicating potential involvement of UBE3B in the regulation of neuronal signaling in the brain (Basel-Vanagaite et al. 2012). The UBE3B protein was also shown to be involved in mitochondrial function and oxidative stress responses in mammalian cells (Braganza et al. 2017). In C. elegans, the oxi-1 gene encodes an ubiquitin ligase homologous to UBE3B (58% amino acid similarity); expression of C. elegans oxi-1 is induced by oxidative stress and is required for proteasomal responses to this stress (Basel-Vanagaite et al. 2012). However, despite the link to neurodevelopmental disorders including BPID, specific roles for UBE3B or oxi-1 in neuronal biology and synaptic function, with or without oxidative stress, have not been explored (Basel-Vanagaite et al. 2012). A second C. elegans gene fshr-1, which encodes a G protein-coupled receptor homologous to a family of mammalian glycopeptide hormone receptors (Cho et al, 2007), is also involved in both oxidative stress responses and neuronal signaling. Specifically, fshr-1 regulates expression of gcs-1, an oxidative-stress response gene (Miller et al. 2015) and was identified in an RNAi interference screen as a gene required for proper structure and function of neuromuscular synapses (Sieburth et al. 2005). Despite data suggesting roles for oxi-1 UBE3B, and fshr-1 in neuronal signaling, which is susceptible to oxidative damage, neither gene has been investigated with regards to neuronal signaling in the presence of oxidative stress. Here, we tested the requirements of both oxi-1 and fshr-1 for their effects on neuromuscular signaling under both normal and oxidative stress conditions in C. elegans.
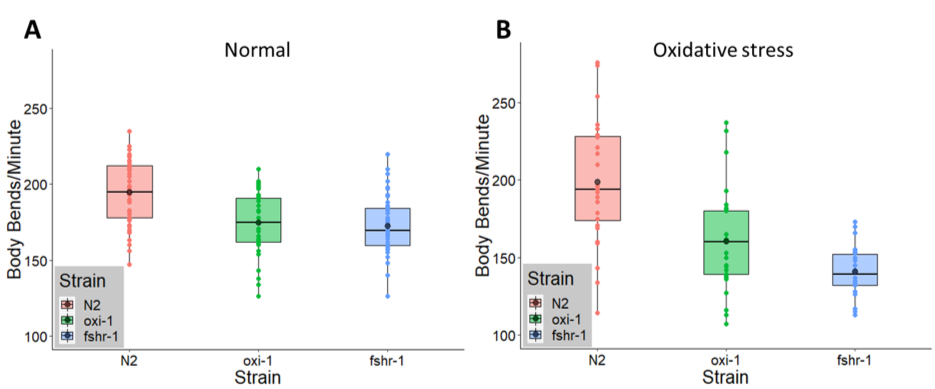
Neuronal signaling activity can be measured at the neuromuscular junction in C. elegans, a model synapse, by observing the motility of individual worms in liquid medium in a body bending assay (Nawa and Matsuoka 2012). Under normal conditions, C. elegans mutants lacking expression of either oxi-1 or fshr-1 had reduced motility compared to wild type animals by 11.0% (p < 0.0001= 2.1167E-05) and 11.2% (p < 0.00001= 3.8259E-06), respectively (Figure 1A). Following exposure to oxidative stress conditions (5 mM paraquat) for 48 hours, the mean number of body bends for wild type animals remained constant (normal: 194 body bends/minute, oxidative stress: 199 body bends/minute). However, the motility of oxi-1 mutants compared to wild type N2 worms under oxidative stress conditions in the body bending assay decreased from 173 to 160.5 body bends/minute, a 19.2% reduction compared to the wild type strain (p < 0.001= 1.77E-04) (Figure 1B). An even larger decrease in body bends, from 172.5 to 140.8 body bends/minute, a decrease of 29.2% (p = < 0.00000012.1771E-08) was observed for fshr-1 mutants compared to wild type animals under oxidative stress conditions (Figure 1B). Successful induction of oxidative stress was determined for each body bending assay by assessing whether a separate strain of worms (ldIs3) carrying the oxidative stress responsive reporter gcs-1p::GFP exposed to paraquat alongside oxi-1, N2, and fshr-1 expressed green fluorescence when observed under a fluorescence stereomicroscope.
Methods
Request a detailed protocolDuring the body bending assay, young adult worms of each strain were individually isolated on unspotted NGM agar plates, and each worm was allowed to move around for 1-2 minutes to dislodge any chunks of bacteria from its body. Then, the worm was placed into its own well containing 100 µL of M9 salt buffer and allowed one minute for acclimation. Afterwards, observation of the worm’s number of body bends over the course of one minute was recorded. Note that a single body bend is defined by the movement of the worm’s head and tail from its initial position to one side and then back (Nawa and Matsuoka 2012).
Using the recorded body bending rates of each strain under both normal and oxidative stress conditions, normality of the data distribution for each strain was first checked using a normal probability plot and/or applying the central limit theorem when sample size was greater than or equal to thirty. Once all datasets were confirmed to be normally distributed, the mean and standard deviation were computed in Microsoft Excel. An F test for equality of variances for N2 compared to each mutant strain under each condition was performed. Statistical significance of the differences in mean body bends between N2 and each mutant strain was determined using two-tailed Student’s t-tests assuming equal variance for all pairs except for N2 vs. oxi-1 under oxidative stress, for which the t test was done assuming unequal variance based on the results of the F test (p = < 0.00017001). For graphical data representation, a boxplot and dot plot were layered on top of each other, including a black dot to represent the distribution mean for each strain, using the ggplot2 function in R.
Reagents
Strains: N2, RB1176 oxi-1(ok1217), RB911 fshr-1(ok778), LD1171 lIdIs3 [gcs-1p::GFP + rol-6]. All strains are available at the CGC.
Materials: M9 buffer, Paraquat (methyl viologen dichloride hydrate, Sigma-Aldrich #856177), NGM agar, OP50 Escherichia coli, 35 mm petri dishes, 96 micro-well plate
Worm Growth Conditions: All strains were grown at constant 20 º C; for assays, worms were plated at the L3/L4 stage onto 35mm NGM agar plates +/- a final paraquat concentration of 5 mM, and grown until the young adult stage at which time body bending assays were performed.
References
Funding
2017-2018 Indiana Academy of Science Junior Research Grant to B.W.; Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Reviewed By
Maureen PetersHistory
Received: August 14, 2018Accepted: August 28, 2018
Published: August 28, 2018
Copyright
© 2018 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Wei, B; Kowalski, JR (2018). oxi-1 and fshr-1 are required for neuromuscular signaling under normal and oxidative stress conditions in C. elegans. microPublication Biology. 10.17912/pfyw-ft85.Download: RIS BibTeX