Description
As neurons mature, responses to γ-aminobutyric acid (GABA) are switched from excitation to inhibition. This excitatory-to-inhibitory GABA functional switch is triggered by GABA-induced depolarization of neurons (Ganguly et al. 2001). Such GABA-induced neuronal depolarization upregulates the expression of chloride exporters, which leads to a decrease in intracellular chloride concentration, but the detailed mechanisms still remain unknown.
In Caenorhabditis elegans (C. elegans), several chloride exporters have been described (Tanis et al. 2009; Bellemer et al. 2011). Among them, abts-1 encodes a sodium-driven chloride-bicarbonate transporter and is expressed in many neurons, including hermaphrodite-specific neurons (HSNs) that regulate egg-laying behavior in mature hermaphrodites. Thus, abts-1 mutants show a resistant phenotype in GABA receptor (GABAR) agonist-induced inhibition of egg laying because of a defect in the GABA functional switch (Bellemer et al. 2011).
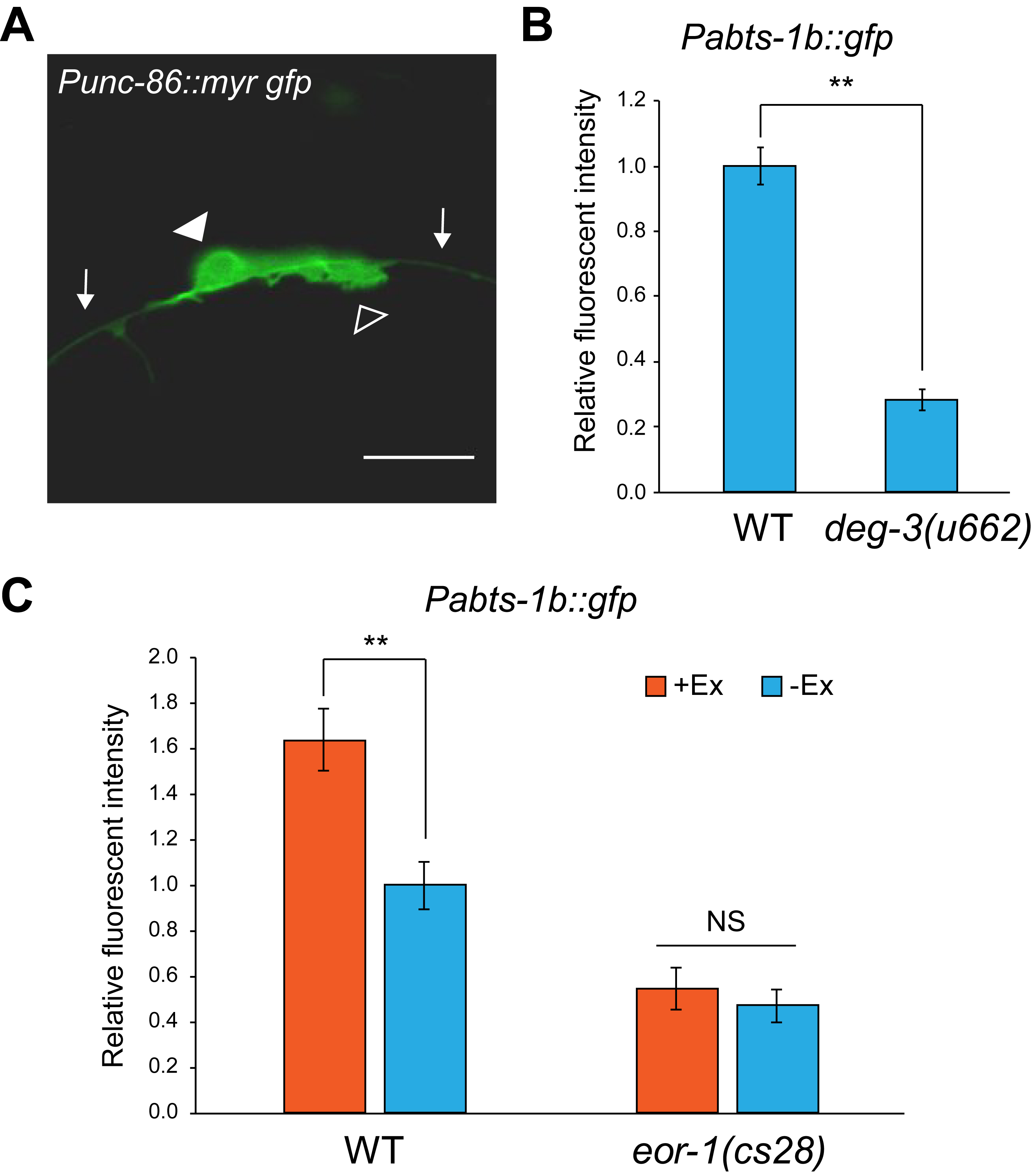
In the present study, we investigated whether abts-1 expression in HSNs is dependent on neuronal activities through synaptic transmission. abts-1 is expressed in HSNs from the L4 larval stage onward (Shinkai et al. 2018). To measure abts-1 expression levels in HSNs, we used transgenic worms carrying vsIs138 [Pabts-1b::gfp, lin15(+)]. Quantitative fluorescent measurements were performed as previously described (Shinkai et al. 2018). Since presynaptic inputs are important for the GABA functional switch, we investigated the effect of PLM neurons, which innervate to HSNs (White et al. 1986). Importantly, PLM touch receptor neurons show contact with HSNs during HSN development (Figure 1A), suggesting that they may have a possible role in neuronal maturation of HSNs. Therefore, we measured the expression level of Pabts-1b::gfp in HSNs of deg-3(u662) mutants, which lose touch sensory neurons including PLM neurons at the L2-L3 larval stage because of abnormal cell death (Treinin and Chalfie 1995). Pabts-1b::gfp expression was significantly reduced in HSNs of deg-3(u662) mutants (Figure 1B), suggesting that presynaptic inputs from PLM neurons are required for the expression of Pabts-1b::gfp. Next, we investigated the effect of increased presynaptic input from PLM neurons. The gain-of-function mutation of pkc-1 has been previously used to enhance presynaptic activities through an increase of both synaptic and dense-core vesicle release from neurons of interest (Sieburth et al. 2005; Sieburth et al. 2007; Shinkai et al. 2011; Inoue et al. 2013). Compared to wild-type worms, the transgenic worms expressing pkc-1(gf) in PLM neurons showed increased expression of Pabts-1b::gfp in HSNs (Figure 1C). These results suggest that synaptic inputs from presynaptic neurons including PLM modulate the expression of Pabts-1b::gfp in HSNs in a non-cell autonomous manner. We recently reported that EOR-1 transcription factor is involved in the chromatin-state regulation of abts-1b promoter region at an early developmental stage, thus preparing for subsequent gene expression for the maturation of HSNs (Shinkai et al. 2018). To uncover the relationship between chromatin alteration by EOR-1 and presynaptic input-dependent regulation of Pabts-1b::gfp expression during HSN neuronal maturation, we analyzed whether the effect of Pmec-7::pkc-1(gf) on Pabts-1b::gfp expression is altered in eor-1 mutants. eor-1 mutants did not show enhancement of Pabts-1b::gfp expression by pkc-1(gf) (Figure 1C), suggesting that EOR-1 is required for non-cell autonomous regulation of the Pabts-1b::gfp expression in HSNs.
In summary, we provided evidence for activity-dependent regulation of abts-1 gene expression during HSN neuronal maturation and propose that EOR-1-mediated chromatin alterations in the abts-1 promoter may be prerequisites for non-cell autonomous regulation of abts-1 gene expression in HSNs.
Reagents
The strains used in this study were as follows:
Wild-type strain N2, eor-1(cs28), deg-3(u662), LX1514 (vsIs138 [Pabts-1b::gfp, lin-15(+)]), and CX5974 (kyIs262 [unc-86::myr gfp + Podr-1::rfp]).
All strains were cultured on NGM plates with E. coli strain OP50 as a food source under standard conditions.
The transgenes used in this study were as follows:
vsIs138 [Pabts-1b::gfp, lin-15(+)], Ex[Pmec-7::pkc-1(gf) SL2 mCherry, Pmyo-2::gfp], and kyIs262 [unc-86::myr gfp + odr-1::rfp].
Acknowledgments
We thank M. Koelle for providing us with the C. elegans strain LX1514 and T. Ishihara for providing us with plasmids. We also thank WormBase and the Caenorhabditis Genetics Center for supplying us with the C. elegans strains used in this study.
References
Funding
No external funding source
Reviewed By
Derek SieburthHistory
Received: June 9, 2018Accepted: July 10, 2018
Published: July 12, 2018
Copyright
© 2018 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Shinkai, Y; Doi, M (2018). EOR-1 mediates non-cell autonomous regulation of abts-1 gene expression in HSNs. microPublication Biology. 10.17912/pm1b-9z95.Download: RIS BibTeX