Description
The relatively simple nervous system and facile genetics of Caenorhabditis elegans makes it a potential model organism to study the genetic basis of complex neural diseases, such as autism spectrum disorder (ASD). Our previously published study identified conserved human ASD-missense variants in C. elegans orthologs that have a role on morphology, locomotion or fecundity, suggesting C. elegans as an efficient phenotypic model to study conserved ASD variants (Wong et al., 2019). One of the ASD-associated genes screened in this study is daf-18, an ortholog of PTEN. DAF-18/PTEN is a lipid phosphatase protein that dephosphorylates PIP3, a critical lipid second messenger that mediates downstream kinase cascade signaling of the insulin pathway, that in turn regulates expression of genes involved in C. elegans lifespan and dauer formation (Murphy and Hu, 2013). It is reported that the daf-18(e1375) strain, which has a 30–base pairs insertion in the fourth exon, has a dauer defective phenotype (Ogg and Ruvkun, 1998); however, the effect of daf-18/PTEN single amino acid substitution in dauer formation has not been evaluated before. Here, we investigate the dauer entry abilities of previously published ASD-associated daf-18/PTEN missense variants (Wong et al., 2019).
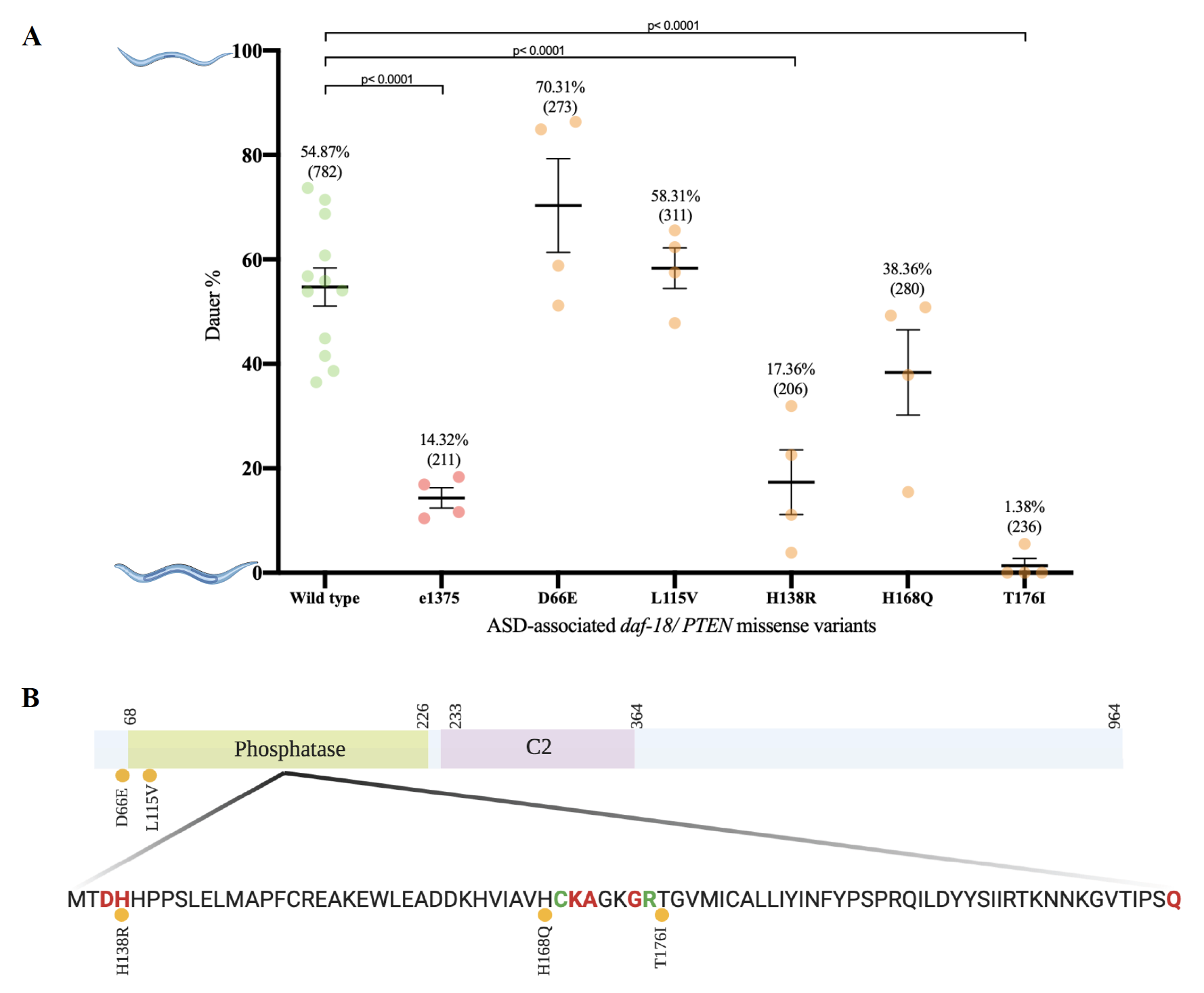
We performed a dauer-entry assay using crude pheromone as a proxy for high population density and heat-killed bacteria as a limited food source. We first determined the crude pheromone concentration required to induce 50% of wild type (N2) strain to enter dauer stage (EC50). Using the calculated EC50value we evaluated each mutant strain in two independent experiments (Table 1). To control day-to-day variations caused by environmental conditions, N2 controls were used in every trail. Additionally, we used the previously reported daf-18 dauer-defective strain (e1375) as a control (Ogg and Ruvkun, 1998). To analyze the obtained data, we performed one-way ANOVA followed by multiple comparisons test (Dunnett test) (Figure 1A).
Table 1 Evaluated ASD-associated daf-18/PTEN mutant strains.
| Strain name | PS7439 | PS7432 | PS7436 | PS7430 | PS7434 |
| C. elegans allele name | daf-18(sy879) | daf-18(sy887) | daf-18(sy881) | daf-18(sy885) | daf-18(sy882) |
| C. elegans protein change | D66E | L115V | H138R | H168Q | T176I |
| Human protein change | D22E | L70V | H93R | H123Q | T131I |
As expected, we observed statistically significant difference between our wild type and dauer defective control (e1375). Our results show that H138R (PS7436) and T176I (PS7434) daf-18 mutant strains have a significant difference from the wild type control, and a non-significant difference from the dauer defective control, suggesting these missense alleles cause dauer defective phenotypes to the same level of the e1375 strain. In contrast, we found the D66E (PS7439), L115V (PS7432) and H168Q (PS7430) mutant strains are significantly different from the dauer defective control and not significantly different from the wild type control, suggesting these three missense alleles do not cause the same level of dauer defective phenotype as the defective control does. Altogether, our current data show that H138R and T176I are defective in dauer entry, while D66E, L115V and H168Q daf-18 mutant strains are not (Figure 1A).
The Conserved Domain Database (Conserved Domain Search) of NCBI (ID: G5EE01) predicts that four of the five missense variations evaluated in this study are located in the phosphatase domain of DAF-18/PTEN(Figure 1B). It is predicted that amino acid Histidine 138 is located in the active site of the protein, suggesting that H138R (PS7436) amino acid change might have an impact on PIP3 substrate specificity, which could explain the dauer defect phenotype observed. Moreover, it is predicted that both amino acid Histidine 168 and Threonine 176 are located close to the catalytic site of the phosphatase domain. We found a dauer defective phenotype resulting from a T176I substitution (PS7434) but not from a H168Q amino acid substitution (PS7430).
These data suggest that certain amino acids in the catalytic and active sites of DAF-18/PTEN decrease the function of the protein, in turn regulating C. elegans dauer development. Future evaluation of PIP3 phosphorylation levels could confirm the effect of these variations on the phosphatase activity. The results of this study contribute to the knowledge of the mechanisms controlling C. elegans dauer entry, and to the C. elegans phenotype-screening of conserved ASD variations.
Reagents
Genotyping missense variants
To confirm ASD-associated daf-18/PTEN missense variations we genotyped each previously generated strain as follows: about 10 young worms were picked into 5 μL lysis buffer (50 mM KCl, 10 mM Tris pH 8.3, 2.5 mM MgCl2) with proteinase K (20 mg/mL; Invitrogen™) and incubated at 65°C for 10 minutes and 85°C for 1 minute. The genomic DNA was amplified by PCR reaction and then treated with a restriction enzyme (New England Biolabs® Inc.) as described in Table 2.
| Strain | Forward primer | Reverse primer | Enzyme | Wild type | Mutants |
| PS7439 |
5’-CCCAATGGTTACTCCTCCTC-3’ |
5’-CCTGTGTTAGTCCTCCTTCAAT-3’ |
SacI | 611 bp | 202 bp, 409 bp |
| PS7432 |
5’-CCCAATGGTTACTCCTCCTC-3’ |
5’-CCTGTGTTAGTCCTCCTTCAAT-3’ |
SacII | 611 bp | 210 bp, 401 bp |
| PS7436 |
5’-CCCAATGGTTACTCCTCCTC-3’ |
5’-CCTGTGTTAGTCCTCCTTCAAT-3’ |
AvaI | 611 bp | 135 bp, 476 bp |
| PS7430 |
5’-TGTCCGACCCGTTAGTCTTC-3’ |
5’-CCACGTAGACACCAATCAACT-3’ |
HpyCH4V | 265 bp | 55 bp, 210 bp |
| PS7434 |
5’-CTGTCCGACCCGTTAGTCTTC-3’ |
5’-CCACGTAGACACCAATCAACT-3’ |
ApaL1 | 340 bp | 150 bp, 190 bp |
Dauer-entry assay on pheromone plates
The crude pheromone used was extracted from exhausted liquid culture medium, resuspended with distilled water, and stored at −20 °C (Butcher et al., 2007). The day before each experiment, NGM pheromone plates were freshly prepared and dried overnight at room temperatureand young C. elegans hermaphrodites of each strain were picked and incubated at 25°C overnight. On the day of the experiment, ∼10 young adults of each strain were placed onto each pheromone plate with 2 µL of OP50 and allowed to lay ∼70-80 eggs before being removed. We added 18 µL of heat-killed OP50 to each plate. After 48 hours of incubation at 25.5 °C, dauers and non-dauers were counted on each plate (Lee et al., 2017).
References
Funding
CGC: Simons Foundation SFARI pilot grant and summer fellowship.
MC: NIH-F32 fellowship (1 F32 GM131570-01)
WRW, CC, PWS: NIH U01-NS111697.
Reviewed By
AnonymousHistory
Received: September 30, 2019Accepted: October 8, 2019
Published: October 16, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
González-Cavazos, C; Cao, M; Wong, WR; Chai, C; Sternberg, PW (2019). Effects of ASD-associated daf-18/PTEN missense variants on C. elegans dauer development. microPublication Biology. 10.17912/micropub.biology.000177.Download: RIS BibTeX