Touro University California, Vallejo, California, 94945 USA
Description
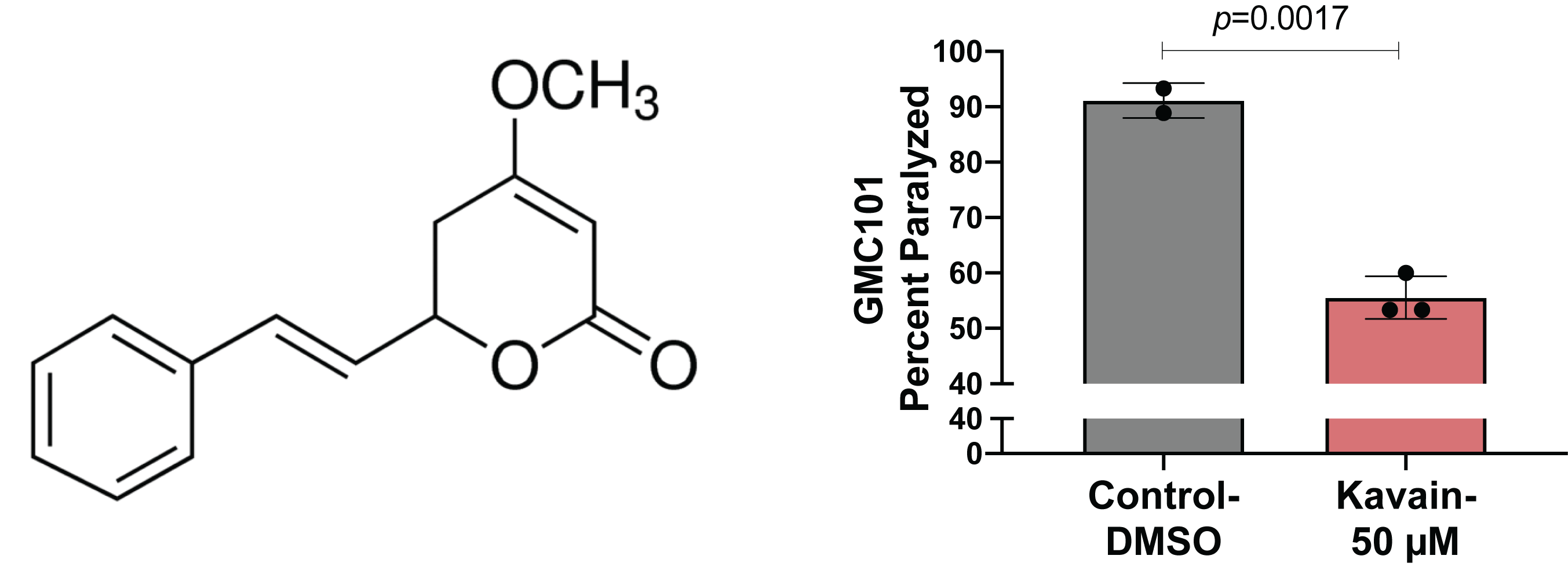
Kavain belongs to a group of lactone-based compounds collectively known as kavalactones, present in the pepper plant kava (P. methysticum). Kavalactones have been shown to possess diverse biological activities including sedation and anxiolysis (Ooi et al., 2018). Kavain in particular has been demonstrated to show potent anti-inflammatory properties in various in vitro and animal models (Guo et al., 2018; Singh et al., 2018; Tang and Amar, 2016; Yuan et al., 2011). A study in C. elegans reported that kavain increases lifespan by inhibiting advance glycation end-products (AGEs), which are known to suppress lifespan (Chaudhuri et al., 2016; Upadhyay et al., 2014). Another study reported that kavain increases acetylcholine (ACh) transmission at the neuromuscular junction (Kautu et al., 2017). Since loss in ACh transmission and increased formation of AGEs are closely linked to Aβ-pathology, we hypothesized that kavain may protect against Aβ-induced toxicity (Kar et al., 2004; Li et al., 2013). We tested kavain in the C. elegans GMC101 strain that over-expresses human Aβ in body wall muscle cells (McColl et al., 2012). Kavain at a concentration of 40 and 80 μM was shown to increase lifespan, thus we decided to use a dose between these ranges (Upadhyay et al., 2014). We observed GMC101 animals fed 50 μM kavain showed significantly less paralysis when shifted to the higher permissive temperature (25o C). The result shows that kavain suppresses Aβ-induced proteotoxicity.
Methods
Request a detailed protocolNGM agar plates (35 mm) were prepared under sterile conditions in a laminar flow hood at room temperature (22oC). To these plates, 100 μl of E.coli OP50 was added to form a circular bacterial lawn on the center of each plate. Plates were then left inside the hood (lid closed) for drying. A 100 mM stock of compound was prepared in 100% DMSO and stored in small aliquots at -20°C. From the stock solution, 130 µl of the working drug (50 μM) or control-DMSO (0.05%) solution was prepared by mixing 1.5 μl of stock solution with 130 µl of sterile water and adding to the top of the 35 mm NGM plates (with 3 mL NGM agar) 48 hours post-bacterial seeding. Compound was distributed over the entire plate surface and allowed to dry in a sterile hood with the lid open for at least 1 hour. Plates were then allowed to sit at 20°C for 24 hours before use.
Egg-lay synchronized populations of GMC101 animals were grown from eggs at 20o C until the L4 larval stage and then transferred to fresh 35 mm plates treated with Control-DMSO plates or kavain-50 μM. Plates were immediately shifted to 25oC and paralysis was scored 48 hours after the temperature shift. Animals were scored as paralyzed if they failed to move during observation and exhibited ‘halos’ of cleared bacteria around their heads (indicative of insufficient body movement to access food), eggs accumulated close to the body, or if they failed to respond to touch-provoked movement with a platinum wire.
Reagents
GMC101: dvIs100 [unc-54p::Aβ-1-42::unc-54 3′-UTR + mtl-2p::GFP]. [unc-54p::Aβ-1-42] expresses full-length human Aβ1-42 peptide in body wall muscle cells that aggregates in vivo. Kavain: Sigma Aldrich (#59780).
Acknowledgments
C. elegans strain used in this work was provided by the Caenorhabditis Genetics Center (CGC), funded by the NIH Office of Research Infra- structure Programs (P40OD010440). MC is supported by the Larry L. Hillblom Foundation.
References
Funding
R01AG029631
Reviewed By
Kim CaldwellHistory
Received: May 3, 2020Revision received: May 18, 2020
Accepted: May 19, 2020
Published: May 21, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Chamoli, M; Chinta, SJ; Andersen, JK; Lithgow, GJ (2020). Kavain suppresses human Aβ-induced paralysis in C. elegans. microPublication Biology. 10.17912/micropub.biology.000254.Download: RIS BibTeX