Description
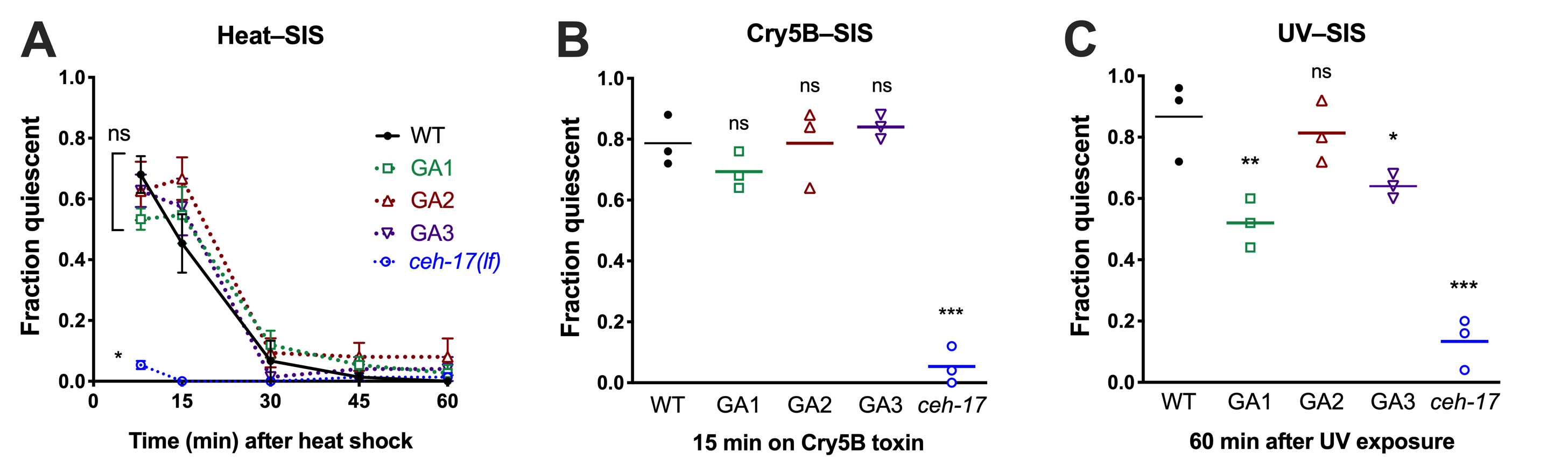
Across species, sleep is increased following exposure to damaging conditions, a phenomenon known as stress-induced sleep (SIS) (Hill et al. 2014; Lenz et al. 2015; Zada et al. 2019). In C. elegans, SIS is dependent on the ALA interneuron (Hill et al. 2014; Nelson et al. 2014), which promotes a coordinated quiescent state through the collective action of several neuropeptides (Nelson et al. 2014; Nath et al. 2016). Recently it has been shown that ALA ablation can suppress phenotypes associated with loss of the cephalic sensilla sheath (CEPsh) glia, such as prolonged larval development and locomotor pausing in adults (Katz et al. 2018), indicating that these glia attenuate certain aspects of ALA function. The CEPsh glia form a tubular structure surrounding the sensory endings of the CEP neurons and also extend thin processes that sheath the nerve ring, including the synapse between ALA and the postsynaptic AVE, a major locomotor interneuron. While the ALA is likely to promote SIS via peptidergic rather than synaptic signaling (Nelson et al. 2014; Nath et al. 2016), we wished to determine whether CEPsh glia ablation may impact the SIS-promoting function of ALA. To this end, we examined three independent lines that genetically ablate the four CEPsh glial cells (Katz et al. 2018), and compared the SIS responses of these strains to wild type and to ALA specification-defective ceh-17(np1) mutant animals. We examined three conditions known to trigger ALA-dependent sleep: noxious heat, pore-forming Cry5B toxin, and ultraviolet light exposure (Figure 1). We found that while the glia-ablated (GA) lines displayed a trend toward enhanced heat-SIS at the 15min time point, there were no significant differences in heat-SIS between the GA lines and wild-type. The GA lines also showed wild-type Cry5B-SIS. Surprisingly, two of the GA lines showed reduced UV-SIS, rather than the enhanced sleep that would be predicted if the CEPsh glia attenuate ALA’s peptidergic function. Our data indicate that CEPsh glia are largely dispensable for stress-induced sleep, but may modulate the UV–SIS response. As UV exposure elicits a delayed sleep response relative to other SIS triggers, we speculate that UV damage may activate ALA in a manner that is distinct, and partially supported by glial function.
Methods
Request a detailed protocolCEPsh glia were ablated at the L1 stage by expression of a reconstituted caspase-3 gene from the hlh-17 promoter (Katz et al. 2018). Behavior was assessed in well-fed (on E. coli OP50) pre-fertile adult animals on NGM agar plates. Quiescence was defined as complete immobility and lack of pharyngeal pumping during a 5 second observation under a stereomicroscope at 375x magnification with experimenter blind to genotype. We observed that immobility was accompanied by feeding quiescence in all cases, but not vice versa, i.e., ‘fraction quiescent’ is equivalent to ‘fraction immobile’. For heat-SIS, 12 ml agar plates were sealed with parafilm and placed upright in a 37°C water bath for 11 minutes, then placed on ice for 2 minutes to return them to room temperature (Goetting et al. 2018). For Cry5B-SIS, animals were placed onto NGM plates containing Cry5B-expressing bacteria (Hill et al. 2014) and examined for SIS 15 minutes later in the presence of Cry5B. For UV-SIS, plates were placed lid-side down on a 302 nm 60 mW ultraviolet (UVB) light source for 50 seconds and examined 60 minutes later for SIS, as UV-induced sleep takes longer to set in than other forms of SIS (DeBardeleben et al. 2017; Goetting et al. 2018).
Reagents
Wild-type N2 and IB16 ceh-17(np1) from the CGC; OS3537 (GA1) nsIs168 (Phlh-17::recCaspase-3, Punc-122::GFP, Pptr-10::myrRFP), OS3540 (GA2) nsIs171 (Phlh-17::recCaspase-3, Punc-122::GFP), and OS3549 (GA3) nsIs180 (Phlh-17::recCaspase-3, Punc-122::GFP) from M. Katz; Cry5B toxin from Raffi Aroian. All reagents are available from our lab.
Acknowledgments
Many thanks to Menachem Katz for strains and for helpful comments on the manuscript.
References
Funding
C.V.B. is supported by the National Science Foundation Faculty Early Career Development Program (IOS#1553673). The Caenorhabditis Genetics Center (CGC) is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD-010440).
Reviewed By
David RaizenHistory
Received: May 6, 2020Revision received: May 28, 2020
Accepted: May 30, 2020
Published: May 30, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Soto, R; Van Buskirk, C (2020). The C. elegans CEPsh glia are largely dispensable for stress-induced sleep. microPublication Biology. 10.17912/micropub.biology.000261.Download: RIS BibTeX