Description
The action potential (AP) is the basic signaling unit in various crucial physiological processing, for instance, in neurotransmission, muscle contraction, and glandular secretion (Koch, 1990). The classic model animal, Caenorhabditis elegans (or C. elegans), with a simple and compact nervous system, conservatively employs the calcium-mediated all-or-none APs for odor response in AWA olfactory neurons (Liu et al., 2018), as well as for muscle contraction in body wall muscles (Gao and Zhen, 2011; Liu et al., 2011) and pharyngeal muscles (Davis et al., 1999). Plateau potentials were also observed in ASE and RMD neurons (Goodman et al., 1998; Mellem et al., 2008; Lockery et al., 2009; Lockery and Goodman, 2009), though the underlying roles in specific behavior are still elusive. Either in neurons or in muscles, the action potential firing is dependent on the excitatory pre-synaptic vesicles release. The minimum number of the presynaptic vesicles to elicit a single action potential in C. elegans has not been reported before. Here, by the combination of optogenetics with in-vivo patch clamping technology, we demonstrated that at least approximately 37 excitatory acetylcholinergic vesicles are required for the initiation of an action potential at post-synaptic body wall muscles.
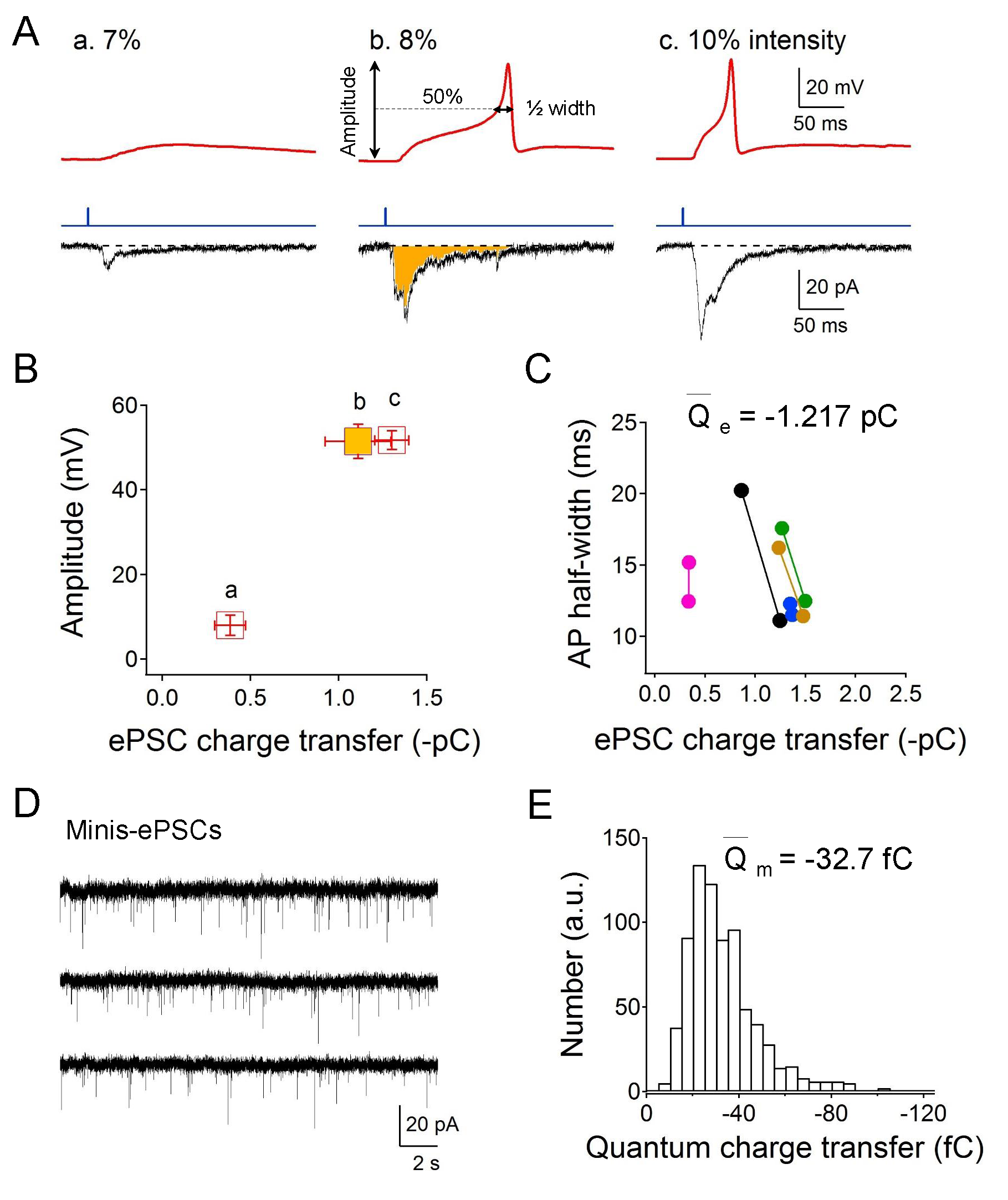
We used the transgenic strain ZX460 with zxIs6 that expresses the optogenetic protein, ChR2 (H134R), in all excitatory cholinergic motor neurons. By gradually increasing the irradiated blue light intensity (from 1-10% of the full intensity 8 mW/mm2), graded electrical responses and eventually all-or-none APs were evoked at the neuromuscular junction. Graded increased excitatory postsynaptic currents (EPSCs) were also recorded. The used holding potential of -30 mV is the reversal potential of postsynaptic ionotropic GABA receptor and therefore the GABAergic inhibitory PSCs could not be recorded (Gao and Zhen, 2011; Maro et al., 2015). Specifically, as the light intensity reached 8% of the full intensity (~0.64 mW/mm2), solid action potentials could be evoked (Fig 1A). Consistent with the all-or-none principle, the amplitude of the evoked muscular action potentials was no longer changed even with stronger light stimulation (Fig 1B, bc). The AP half-width, however, was slightly shortened and was correlated with higher EPSCs charge transfer (Fig 1C). The average smallest EPSCs quantity of electric charge is -1.217 pC for the elicitation of an action potential in muscle cells. To evaluate the single vesicle charge transfer, we measured miniature-EPSCs holding at -30 mV (Fig 1D). The average miniature-EPSCs quantity of electric charge is -32.7 fC (Fig 1E), thus the calculated minimum number of cholinergic vesicles is 37 by dividing the quanta charge into the smallest EPSCs charge.
Methods
Request a detailed protocolStrains and Culturing Conditions
Strain ZX460: zxIs6 [Punc-17::ChR2(H134R)::YFP + lin-15(+)] was cultured in the dark at 22℃ on OP50-seeded Nematode Growth Medium (NGM) plates (Brenner, 1974). Plates containing all trans-retinal were prepared by spreading 300 μl of OP50 culture mixed with 0.25 μl of 100 mM all trans-retinal stock (dissolved in ethanol) (Liewald et al., 2008).
Electrophysiology
The dissection of C. elegans was described previously (Richmond et al., 1999, Gao and Zhen, 2011). Briefly, one-two days old hermaphrodite adults were glued to a sylgard-coated cover glass covered with bath solution. Animals were immobilized on Sylgard® 184 Silicone Elastomer (Dow Corning)-coated glass coverslips using tissue adhesive glue (Histoacryl® Blue, Braun). The curved dorsal side of the animal was dissected using sharpened tungsten or glass needle. After clearing the viscera by suction through a glass pipette, the cuticle flap was turned and gently glued down using WORMGLU (GluStitch Inc.) to expose the neuromuscular system. The muscle cells were patched using fire-polished 4−6 MΩ resistant borosilicate pipettes (World Precision Instruments, USA). Membrane potential and currents were recorded in the whole-cell configuration by a Digi-Data 1440A and a MultiClamp 700A amplifier using the Clampex 10 software, and data were processed with Clampfit 10.2 (Molecular Devices). Data were digitized at 10 kHz and filtered at 2.6 kHz. Light stimulation of zxIs6 was performed with an LED lamp (KSL-70; RAPP OptoElectronic) at a wavelength of 470 nm (full intensity 8 mW/mm2), controlled by the Axon amplifier software.
In this study, each cell recording is from one animal unless otherwise noted. ‘n’ means the recorded animal number. Usually, three membrane potentials, and three EPSCs data evoked by different light intensities (7%, 8%, 10%) from a muscle cell were collected and analyzed.
The recording solutions used in this study: the pipette solution contains (in mM) K-gluconate 115; KCl 25; CaCl2 0.1; MgCl2 5; BAPTA 1; HEPES 10; Na2ATP 5; Na2GTP 0.5; cAMP 0.5; cGMP 0.5, pH7.2 with KOH, ~320 mOsm. The bath solution consists of (in mM) NaCl 150; KCl 5; CaCl2 5; MgCl2 1; glucose 10; sucrose 5; HEPES 15, pH7.3 with NaOH, ~330 mOsm. Leak currents were not subtracted. All chemicals were from Sigma. Experiments were performed at room temperatures (20−22℃).
Statistical Analysis
Data analysis and graphing were performed using Excel 2013 (Microsoft), Igor Pro 6.21 (Wavemetrics), and Clampfit 10.2 (Molecular Devices). All data are presented as mean ± SEM.
Reagents
ZX460 zxIs6 [Punc-17::ChR2(H134R)::YFP; lin-15+] V.
References
Funding
This work was supported by the National Natural Science Foundation of China (31871069, 31671052), the National Science Foundation of Hubei Province (2018CFA039) to SG.
Reviewed By
AnonymousHistory
Received: June 14, 2020Revision received: October 1, 2020
Accepted: October 2, 2020
Published: October 6, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Chen, L; Wang, Y; Gao, S (2020). Minimum number of synaptic vesicles for the initiation of a single action potential at C. elegans neuromuscular junction. microPublication Biology. 10.17912/micropub.biology.000316.Download: RIS BibTeX