Description
Tetraspanins (TSPAN) are a family of (33 in humans) scaffold proteins consisting of the name-giving four membrane-spanning domains, two extracellular loops, and an intracellular N- and C-terminus. By forming ‘tetraspanin-enriched microdomains’ they participate in organizing the plasma membrane and serve as multipurpose adapters. Some can be found ubiquitously in most cells, while others are specific to certain cell types. On a subcellular level, enrichment in distinct membrane-associated organelles has been documented for several members (Termini and Gillette, 2017).
One member, Cd63, shows such specific enrichment in intraluminal vesicles (ILV) of late endosomes (LE; also called multivesicular bodies, MVB). Further, it is found in exosomes, small extracellular vesicles created from ILV of specialized MVB, which become released after fusion of the MVB with the plasma membrane (Piper and Katzmann, 2007; Pols and Klumperman, 2009; Simons and Raposo, 2009). Another specialized form of LE are melanosomes, which are dedicated for pigment (types of melanin) production in all vertebrates. They are mainly found in two types of tissue, the melanin producing melanocytes (‘melanophores’ in basal vertebrates like amphibians), and in the retinal pigment epithelium (RPE), the outer, light-shielding layer of the vertebrate optic cup (Raposo and Marks, 2007). In amphibians, melanophores derive from trunk neural crest (TNC) cells at the dorsal neural tube, from where they migrate to their lateral, mostly dermal destination during later embryogenesis (Collazo et al., 1993). Cd63 also localizes to early melanosomes, and was shown to be required for melanogenesis in human melanoma cells (Basrur et al., 2003; van Niel et al., 2011).
The externally developing frog Xenopus represents a great vertebrate model system for developmental analyses, especially true for the neural crest and its derivatives. However, neither the tissue-specific developmental expression, nor the functional requirement of a specific TSPAN have been tested in vivo. Therefore, in this study, we analyzed the expression and function of the Xenopus cd63 orthologue during tailbud stages, i.e. when major tissue differentiation processes and organ development take place. We were interested if cd63 showed specific enrichment in melanosome-associated tissues like TNC cells, melanophores or the retinal pigment epithelium (Collazo et al., 1993; Sinn and Wittbrodt, 2013). Further, we tested if loss of cd63 affected development and function of these tissues.
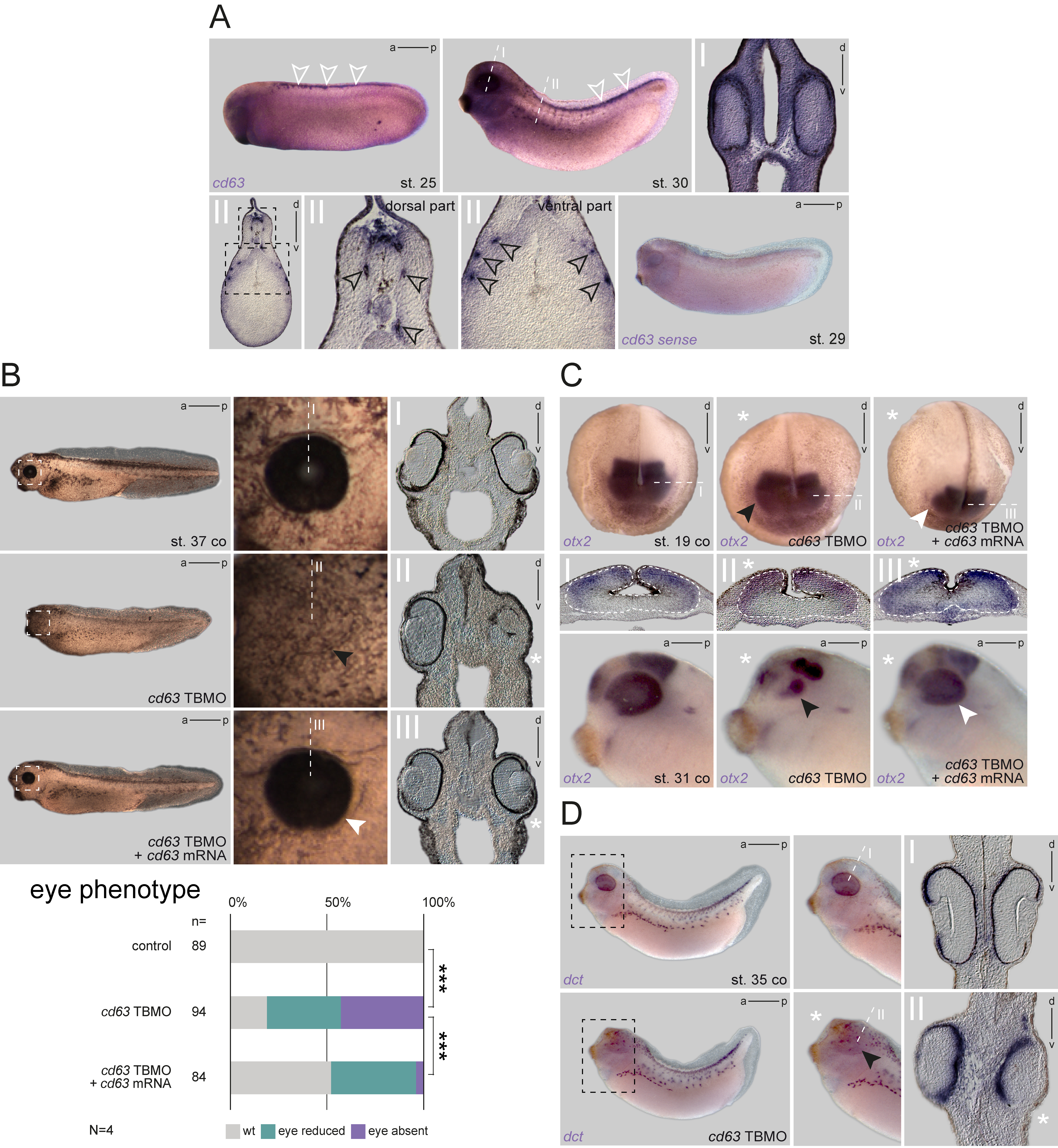
Expression analysis by in situ hybridization (ISH) using a cd63-specific probe revealed a striking specific enrichment of mRNA in certain tissues. At early tailbud stages (Figure 1A; st. 25), cd63 showed strong expression in premigratory TNC cells. This tissue was still positive at later tailbud stage, then localized mRNA signals were also found in the cement gland, head mesenchyme, brain, dermis, dorsal fin mesenchyme, migrating TNC cells and eyes, including the RPE (Figure 1A; st. 30). At this stage, transcript enrichment could also be detected laterally in putative melanophores, i.e. before start of pigment synthesis (Figure 1A). A sense probe control did not detect any signal in these tissues (Figure 1A). These analyses demonstrated that cd63 was expressed in a tissue-specific manner, indicating a spatial requirement for certain embryonic processes. Moreover, the mRNA distribution suggested a potential role in pigment-associated tissues, i.e. melanophores and RPE.
Thus, we next wanted to test a potential functional requirement of Cd63 for melanogenesis in tadpoles. We designed a translation-blocking morpholino oligomer (TBMO) that binds the 5’UTR of both homeologs of the X. laevis cd63 mRNA, to block protein synthesis. Embryos were injected unilaterally into the neural lineage to target the eyes, TNC, and melanophores. When control embryos or internal control side of morphants showed normal eye and melanophore formation, the targeted side of morphants displayed strong defects in eye morphology (Figure 1B). Most morphant halves displayed reduced or lacking eyes when analyzed externally. Transversal sections confirmed defects in optic cup formation, with defects ranging from partial to nearly complete lack of most major structures (RPE, retina and lens). This effect was partially rescued by co-injection of wild type (WT) cd63 mRNA lacking the 5’UTR, i.e. insensitive to the MO, demonstrating specificity of the observed phenotype. Rescued halves showed WT or milder phenotypes (Figure 1B). Interestingly, no obvious phenotype was detected in morphant melanophores. Migration appeared not to be altered, and neither number, nor the shape of melanophores was significantly different from control halves. To understand the temporal basis of this eye phenotype, a subset of injected specimens was fixed at late neurula or tailbud stages to check for expression of otx2, a forebrain marker highlighting eye tissues (optic vesicle/cup or differentiated eyes, respectively). At neurulation, expression was not reduced after cd63 knockdown but revealed alterations in prosencephalon shapes on the injected side, indicating a morphogenetic effect (Figure 1C). Transversal sections of such embryos revealed an inhibition of medial closure of the prosencephalon, and impairment of optic vesicle formation (sections I-III in 1C), together causing widening of the otx2 expression domain on the injected half. Later, when embryos reached tailbud stages, this morphogenetic phenotype appeared more pronounced, resulting in partial reduction of optic otx2 expression. Again, the severity of both phenotypes was milder in rescued embryos.
Finally, some embryos were checked for expression of dopachrome tautomerase (dct), a gene coding for a tyrosinase-related enzyme participating in melanin synthesis in the RPE and in melanocytes, and which is highly expressed in these tissues in Xenopus as well (Kumasaka et al., 2003). As reported before, expression was highly reminiscent of cd63 at tailbud stages (cf. Figure 1A). We did not detect any effect on TNC formation, number or migration ability of melanophore precursors, or expression intensity, indicating correct specification of melanophores (Figure 1D). Using dct to highlight the RPE, embryos showed again a clear reduction in optic tissues, though, sections revealed remnant RPE expression even in severely affected specimen. Thus, knockdown of cd63 did not affect melanophore development in our approach. Altogether, from these analyses we conclude that Cd63 is required for correct eye morphogenesis, but surprisingly dispensable for melanophore specification and melanogenesis in Xenopus.
While the observed phenotypes were shown to be specific to the loss of cd63, we cannot exclude a compensatory effect taking place in the melanophore lineage. In some cases, including Cd63-dependent melanosome formation in mice, it has been shown that the RPE is more sensitive to alterations in the process of melanogenesis as compared to melanocytes, as this process is much more dynamic in the latter (cf. Lopes et al., 2007; van Niel et al., 2011). Nearly no TSPAN gene has ever been analyzed in Xenopus development so far; therefore, the loss of cd63 in melanophores might also be compensated by other TSPAN potentially expressed in these cells as well. Alternatively, in contrast to mouse melanocytes, Cd63 might not directly participate in amphibian melanogenesis (van Niel et al., 2011). It could participate in other cellular processes, not directly related to melanin production, e.g. regulation of exosomes. The lack of impact on migration of TNC derivatives is in agreement with another known role of Cd63, namely its inhibitory effect on cell migration. While known as a factor elevated in early stage melanomas, it has been shown to be a negative driver of advanced stages by inhibiting epithelial-mesenchymal transition, and thus invasive behavior (Radford et al., 1997; Jang and Lee, 2003; Lupia et al., 2014). Therefore, in our knockdown approach, migration behavior of TNC should rather be promoted. Finally, the role of Cd63 in cell migration is tightly associated with its regulatory function of integrin abundance at the plasma membrane, an influence on cell adhesion shared with other TSPAN (Termini and Gillette, 2017). Concerning the morphogenetic phenotypes we observed in the early tadpole eyes, it is interesting to note that correct integrin localization has been demonstrated to be essential for eye morphogenesis in fish, providing an interesting link to our observations (Martinez-Morales et al., 2009).
Methods
Request a detailed protocolXenopus laevis care and maintenance
Frogs were purchased from Nasco (Fort Atkinson, WI, USA). Handling, care and experimental manipulations of animals was approved by the Regional Government Stuttgart, Germany (V349/18ZO ‘Xenopus Embryonen in der Forschung’), according to German regulations and laws (§6, article 1, sentence 2, nr. 4 of the animal protection act). Female frogs (between 4 and 12 years old) were injected subcutaneously with 300-700 units of human chorionic gonadotropin (Sigma), depending on weight and age, to induce ovulation. Sperm of male frogs was obtained by maceration of testes (stored at 4°C in 1x Modified Barth`s saline with HEPES). Embryos were staged according to Nieuwkoop and Faber (1994). Only clutches of embryos from healthy females were used for the experiments and early embryonic stages were chosen only if survival rates were normal. For experiments, individual embryos from one batch were randomly picked and used as control or tested specimens. If control groups displayed unusual or high percentage of developmental defects later in development, such clutches and experiments were excluded as well, based on empirical judgement.
Morpholino design, mRNA synthesis and microinjections
The cd63 TBMO (sequence: 5’TTCTCCTCTCCAGTAACTTGTAACG 3’) targeting the 5’UTR of both X. laevis homeologs was designed using the S-sequence (5’UTR position -40 to -16 distance from the start), as deposited in gene bank (accession: NM_001087051). For rescue experiments, RFP-cd63/CS2 plasmid (Lee et al., 2007) containing the cd63 coding sequence without 5’UTR was linearized with NotI and transcribed in vitro (Sp6 polymerase) using Ambion message machine kit. Drop size was calibrated to four nl per injection. One pmol of MO was injected unilaterally into the animal hemisphere at four cell stages with or without rescue mRNA (260 pg per injection), targeting predominantly the neural half of the embryo. The opposite side was used as an internal control.
In situ Hybridization
Partial ISH probes for X. laevis cd63 (accession: NM_001087051) and dct (accession: BC073623.1) were cloned using standard RT-PCR with the following primer pairs:
(F) 5’-TTCTTCAACTTCGTGTTCTGG-3’/(R) 5’-CCGTGATATTACTTGTGTTGC-3’ (cd63), and
(F) 5’-GCCGCTGAAGTTCTTTAACTC-3’/(R) 5’-GGTAAAGGTAGCATTCATCAAGG-3’ (dct).
For in situ mRNA detection, ISH was performed after fixation in MEMFA for 2-3h at room temperature and processed following a standard protocols (Sive et al., 2000). RNA in situ probes were transcribed using SP6 or T7 polymerases.
Embryo sections
For vibratome sections (thickness: 30-35 μm), embryos were embedded in a glutaraldehyde-cross-linked gelatin-albumin mix (Embedding medium: 2.2 g gelatine,135 g bovine serum albumin, 90 g sucrose dissolved in 450 ml PBS) and razor blade-sectioned as indicated in whole-mount panels using a Leica VT1000S vibratome.
Photo Documentation
Pictures were taken with a Zeiss SteREO Discovery.V12 (for whole embryos) or an Axioplan2 inverted (for sections) microscope using AxioVision 4.6. Adobe Photoshop CS6 was used for cropping and careful brightness adjustments. All figures were arranged using Adobe Illustrator CS6.
Statistical analysis
Statistical calculations of marker gene expression were performed using Pearson’s chi-square test. *=p<0.05, **=p<0.01, ***=p<0.001 were used for all statistical analyses. ‘N’ represents the number of experiments (i.e. number of biological replicates of batches of embryos from different fertilizations), and ‘n’ the number of embryos analyzed (i.e. number of biological replicates of embryos).
Acknowledgments
We like to thank V. Andre, A. Schäfer-Kosulja and W. Möller for technical assistance, K. Feistel and the Blum Lab members for continuous valuable feedback. The otx2 plasmid was a kind gift of Dr. R. Harland, the RFP-cd63/CS2 of Dr. N. Kinoshita.
References
Funding
J.K. was a recipient of a Ph.D. fellowship from the Landesgraduiertenförderung Baden-Württemberg.
Reviewed By
Thomas Hollemann and AnonymousHistory
Received: August 19, 2020Revision received: October 30, 2020
Accepted: November 23, 2020
Published: November 27, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Kreis, J; Bonß, R; Vick, P (2020). The tetraspanin Cd63 is required for eye morphogenesis in Xenopus. microPublication Biology. 10.17912/micropub.biology.000335.Download: RIS BibTeX