Abstract
Apart from the beneficial roles of pyrogallol in industries, it also tends to produce free radicals that trigger apoptosis in human cells. In this study, we checked the toxic effect of pyrogallol in fission yeast S. pombe cells. We observed that the wild type and wat1/pop3 delete cells were unable to grow on plates containing pyrogallol in a dose-dependent manner. Furthermore, the wat1/pop3 delete cells exhibit higher sensitivity against pyrogallol as compared to wild type cells suggesting that the pyrogallol induces oxidative stress. The exposure to pyrogallol also leads to the production of ROS and affects the sporulation in S. pombe.
Description
Pyrogallol [C6H3 (OH)3] is an organic white water-soluble solid compound that finds several uses. It is used as an ingredient in the hair cosmetic industry (Mazzei et al., 2007), photography (Upadhyay et al., 2010), and as an antipsoriatic drug (Khan et al., 2002). Besides its beneficiary roles in industries and as a consumer product, some previous studies report the toxicity caused by pyrogallol due to its tendency to produce free radicals and trigger apoptosis (Han and Park, 2002). Oxidative stress is mainly the cause of its toxicity and was reported to be carcinogenic and tumorigenic in female and male mice respectively (Mercado et al., 2013). Humans are often exposed to pyrogallol through hair dyes and ingestion of beverages like tea, coffee, and cause deleterious effects if consumed beyond the permissible amount. However, some previous studies also suggest its therapeutic role in inhibiting tumor growth in certain animal models as well as induces G2-M arrest in human lung cancer cells (Yang et al., 2009). Additionally, the protective effect of pyrogallol along with other phenols against the oxidative stress caused due to hydrogen peroxide has been reported in Saccharomyces cerevisiae (Mendes et al., 2015).
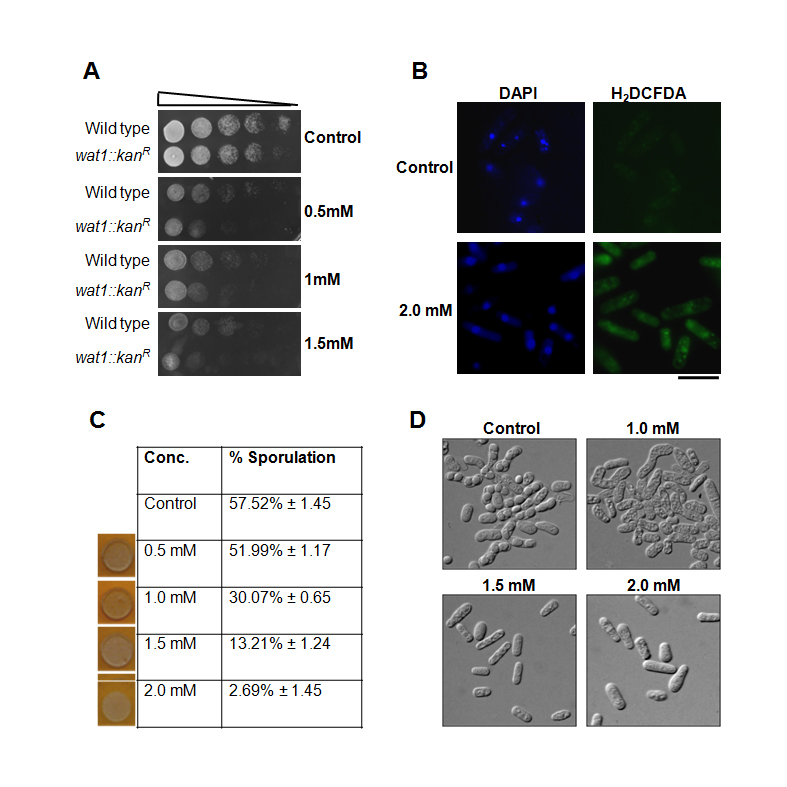
By considering these facts and background information, we examine the effects of pyrogallol treatment in fission yeast. We tested the dose-dependent consequences of pyrogallol on cell viability, sporulation efficacy, and intracellular ROS generation. The toxicity and cell viability parameters were checked in S. pombe at various concentrations by spot assay. We observed that the wild type cells were unable to grow on plates containing pyrogallol in a dose-dependent manner (Fig.1A). Previously, we have shown that the fission yeast wat1/pop3 is required for the oxidative stress response and exhibits sensitivity towards hydrogen peroxide (Ahamad et al. 2016). To further ascertain the role of pyrogallol in oxidative stress response, we checked the pyrogallol sensitivity of wat1/pop3 delete cells. The spotting assay revealed the enhanced pyrogallol sensitivity of wat1/pop3 delete cells as compared to wild type cells (Fig. 1A) suggesting that the pyrogallol induces oxidative stress. Next, we examined the level of reactive oxygen species (ROS) in untreated control and pyrogallol treated wild type cells by staining with H2DCFDA dye which produces green fluorescence in the presence of ROS. We noticed a significant enhancement in green fluorescence under the confocal microscope displayed by pyrogallol treated cells in comparison with control (Fig. 1B) suggesting that the exposure to pyrogallol leads to increased production of ROS. Moreover, it has been reported from the previous studies that at hoisted concentrations, ROS deploys diverse detrimental effects on normal cellular pathways (Azad et al., 2014). Previously we have shown that the increase in ROS due to the inactivation of Wat1/pop3 protein leads to defects in sporulation in fission yeast (Ahamad et al., 2016; Ahamad et al., 2018). To examine the effect of pyrogallol on the sporulation in S. pombe we mixed the wild type cells of opposite mating type on the minimal media plates containing various concentrations of pyrogallol. On control plates, nearly 57.5% asci with four spores were observed indicating the normal sporulation. In contrast, we observed a decrease in the number of asci on the plate containing pyrogallol in a dose-dependent manner. At 1mM and 2mM pyrogallol concentration, the sporulation was reduced to 30% and 2.7% respectively (Fig. 1C & D). Thus, our study demonstrates that oxidative stress is implicated in pyrogallol-mediated toxicity that leads to affect the sporulation in fission yeast Schizosaccharomyces pombe.
Taken together, the present study highlighted the role of pyrogallol inducing oxidative stress response in S. pombe. Alterations in the level of ROS caused by pyrogallol are one of its inherent pro-oxidant properties which cause cell death and sporulation defects. Our results emphasize that it is essential to verify the effects of pyrogallol through various modes to fully understand its mechanism. Hence, further studies are needed to identify the cellular and intracellular targets of this compound.
Methods
Request a detailed protocolGrowth sensitivity assay: For the spotting experiment, wild type and wat1/pop3 delete cells were grown at 25oC up to mid-log phase, 107 cells were serially diluted, spotted on plates containing 0.5mM, 1mM, and 1.5mM pyrogallol. Plates were incubated at 25oC for 3-4 days before taking photograph.
Intracellular ROS detection: Wild type yeast cells were grown till mid-log phase in liquid YEA media and treated with 2mM of pyrogallol for approximately 3 hours. H2DCFDA dye was added and cells were further incubated for 90 min at 30oC to detect ROS by confocal microscope.
Yeast sporulation assay: S. pombe crosses were set up by mixing two strains of opposite mating types on malt extract agar media containing different doses of pyrogallol. The plates were incubated for 3 days at 30oC. The sporulation was monitored by staining the colonies with iodine vapour and also checked under the phase-contrast microscope.
Reagents
Reagents and yeast strains: All the reagents unless specified or mentioned were purchased from HiMedia (India). Pyrogallol and H2DCFDA dye were obtained from Sigma-Aldrich. The stock solution of pyrogallol (10mM) was prepared in water. All the experiments were performed using the haploid strains of S. pombe (SP3: h+leu1-32; SH573: h+ leu1-32 ura4D18 wat1::kanR).
Acknowledgments
We acknowledge Rima Ray Sarkar for the technical help provided in confocal microscopy. NA acknowledges Indian Council of Medical Research (ICMR) and SA acknowledge the University Grant Commission (UGC) for providing research fellowship. The CDRI communication number for this manuscript is 10179.
References
Funding
Science and Engineering Research Board (SERB), India (EMR/2016/000749) and Council of Scientific and Industrial Research, New Delhi, India.
Reviewed By
AnonymousHistory
Received: September 2, 2020Revision received: December 30, 2020
Accepted: January 4, 2021
Published: January 7, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Ahamad, N; Anjum, S; Ahmed, S (2021). Pyrogallol induces oxidative stress defects in the fission yeast S. pombe. microPublication Biology. 10.17912/micropub.biology.000348.Download: RIS BibTeX