The Jackson Laboratory, Bar Harbor, ME USA
Abstract
We have generated a WEE-1.3 strain in C. elegans where we have endogenously tagged the C-terminus with GFP. In this publication we demonstrate that this new strain exhibits the same expression localization pattern as the WEE-1.3 antibody and N-terminally endogenously GFP-tagged WEE-1.3 strain that have been previously published. We also show for the first time that endogenously tagging WEE-1.3 at either termini does not affect the reproductive function of the worms.
Description
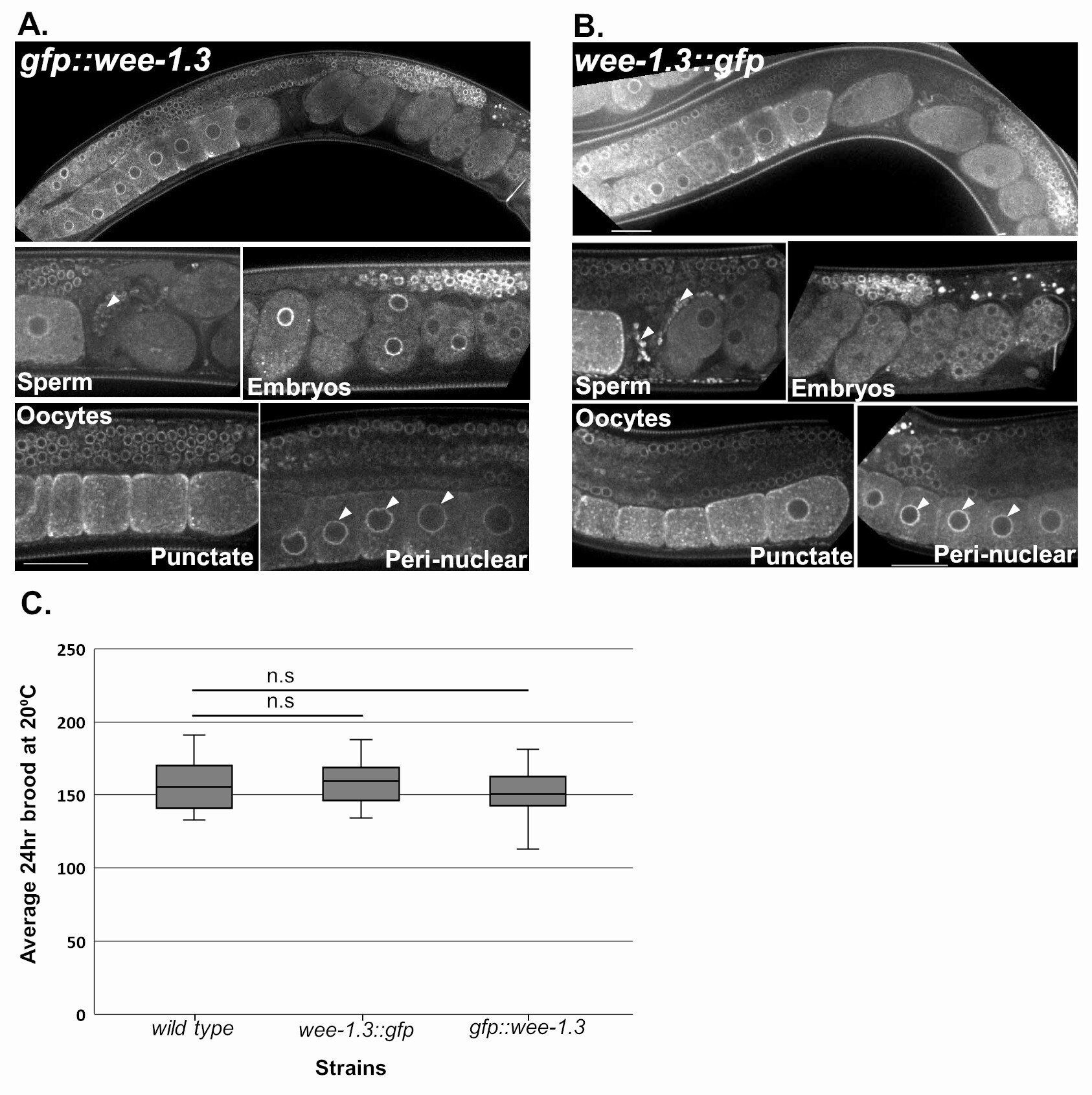
The Wee1/Myt1 family of kinases are important cell cycle regulators during mitosis and meiosis, and act by specifically phosphorylating certain amino acid residues and inhibiting their target genes. The C. elegans WEE-1.3 protein is a member of this family and has been shown to play an essential role in maintaining oocyte meiotic arrest in the nematode (Allen et al., 2014; Burrows et al., 2006). A complete absence of WEE-1.3 results in embryonic lethality, while depletion of WEE-1.3 starting at the L4 larval stage results in precocious oocyte maturation and sterility (Allen et al., 2014; Burrows et al., 2006). Previously we have shown that WEE-1.3 is expressed throughout the soma and germ line of adult hermaphrodites and developing embryos via antibody staining, transgenic animals tagged with GFP at the N-terminus and C-terminus of WEE-1.3, and endogenously tagging the N-terminus of WEE-1.3 via CRISPR (Allen et al., 2014; Fernando et al., 2020). In these situations, the expression of WEE-1.3 is strongly perinuclear, but with cytoplasmic punctae that co-localizes with ER proteins in oocytes and embryos (Allen et al., 2014; Fernando et al., 2020). Here we have generated a homozygous wee-1.3::gfp strain (WDC8) where GFP is inserted at the 3′ end of the wee-1.3 genomic locus via CRISPR/Cas9 endogenous genome editing. The endogenous C-terminal WEE-1.3::GFP strain (WDC8) exhibits a perinuclear and cytoplasmic punctate localization expression pattern that is identical to that observed in the WEE-1.3 antibody stained germ lines, transgenic WEE-1.3::GFP animals and in endogenous gfp::wee-1.3 (WDC2) strains (Figure 1A-B). This expression is ubiquitous throughout the soma, in the germ line from the distal tip to the proximal oocytes, in developing embryos, and in sperm stored in the spermatheca. Importantly, both N- and C-terminally endogenously GFP tagged WEE-1.3 strains have a normal 24-hour brood size compared to wild-type control animals implying that we have not disrupted the function of this important reproductive protein kinase by fusing the GFP protein directly to it (Figure 1C). Our data suggest that the new C-terminally endogenously GFP tagged WEE-1.3 strain represents wild-type WEE-1.3 expression and activity, and can be used to monitor endogenous WEE-1.3 levels and localization appropriately.
Methods
Request a detailed protocolThe wee-1.3(ana8[wee-1.3::gfp]) WDC8 strain was generated via CRISPR/Cas9 genome editing technology following the direct delivery method developed by the Seydoux laboratory (Paix et al., 2017). Superfolder GFP sequence was inserted at the C-terminus immediately upstream of the stop codon (Pédelacq et al., 2006). crRNA30 (5′- atttggatcatcaggcgacg – 3′) (Horizon Discovery Ltd) was used to guide the Cas9 to cut at the 3′ end of the wee-1.3 gene. A PCR repair template was generated using pDONR221 containing Superfolder GFP and wee-1.3 forward (oAKA431) and wee-1.3 reverse (oAKA432) specific primers. oAKA431: 5′- gttttttttccagatgtcatttggatcatcaggcgacgAaGtttccaagggagaggagctctt-3′
oAKA432: 5′ gcaaaaaataaatattcaacaatttttctgatttttgtgcattattacttgtagagctcgtccattc-3′. The bold regions in the primers refer to the Superfolder GFP sequence. The uppercase letters in oAKA431 denote silent mutations that have been introduced to prevent recutting by crRNA30. Screening for successful edits was performed using the co-conversion method and unc-58(e665) as a marker (Arribere et al., 2014). Successful edits were confirmed via PCR using forward primer oAKA88 (5′- gaataatgtgatcgacgaggctcc-3′) and reverse primer oAKA61 (5′- agtgcgatatggtcgagagg-3′). Multiple independent strains were generated, and each strain outcrossed five times before experiments were conducted.
Live imaging was conducted by placing 10-15 worms on a slide containing a 3% agar pad and 10uL of anesthetic (0.1% tricane and 0.01% tetramisole in 1x M9 buffer) then lowering a glass coverslip over the sample. Samples were imaged on a Nikon Ti-E-PFS inverted spinning-disk confocal microscope using a 60x 1.4NA Plan Apo Lambda objective, the 488 laser line, and an Andor iXon 897 EMCDD camera. All image editing was done using NIS-elements software.
For the brood assays, L4 hermaphrodites from each strain (N2, gfp::wee-1.3, wee-1.3::gfp) were placed onto E. coli-seeded MYOB plates and then 16 hours after the L4 stage were singled out (1 worm/plate). The worms were grown at 20ºC, and after 24 hours, the adult mothers were discarded. For all plates, the progeny were counted 1 day after transferring the mother off of the plate. All progeny laid on the plate were counted when determining the brood; this includes eggs and developing larvae. Three independent trials were conducted for a total n ranging from 22-28.
Reagents
N2
WDC2 wee-1.3(ana2[gfp::wee-1.3])
WDC8 wee-1.3(ana8[wee-1.3::gfp])
N2 is available from the CGC, WDC strains are available from the Allen Lab.
References
Funding
This work was supported, in part, by a grant W911NF-18-1-0465 from the Department of Defense to A.K.A. (L.M.F. and A.K.A.) and by grant 1R15HD084253-01A1 from the National Institutes of Health to A.K.A (K.G., R.B. and A.K.A). The spinning disk confocal microscope was acquired through a Department of Defense HBCU/MI Equipment/Instrumentation Grant (#64684-RT-REP) to A.K.A.
Reviewed By
Anonymous and Andrew SingsonHistory
Received: December 10, 2020Accepted: January 9, 2021
Published: January 14, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Fernando, LM; Golliday, K; Boateng, R; Allen, AK (2021). Comparison of N- and C-terminally endogenously GFP-tagged WEE-1.3 strains in C. elegans. microPublication Biology. 10.17912/micropub.biology.000353.Download: RIS BibTeX