Department of Frontier Studies of Medical AI, Tohoku University School of Medicine, Sendai, Miyagi 980-8575, Japan
Department of Life Science, Faculty of Science and Engineering, Setsunan University, Neyagawa, Osaka 572-8508, Japan
Life Science Center for Survival Dynamics, Tsukuba Advanced Research Alliance, University of Tsukuba, Tsukuba, Ibaraki 305-8577, Japan
Laboratory for Developmental Dynamics, RIKEN Center for Biosystems Dynamics Research, Kobe, Hyogo 650-0047, Japan
Abstract
C. elegans spe-9 class genes encode sperm proteins with indispensable roles during fertilization. We have previously reported that spe-45 belongs to the spe-9 class, based on the finding that self-sperm of spe-45(tm3715) hermaphrodites were not consumed by fertilization. In this study, we directly observed live fertilization in the spermatheca of fem-1(hc17) females after mating with spe-45(tm3715) males. As expected, it was clearly shown that spe-45 mutant spermatozoa failed to fuse with the oocyte plasma membrane. Thus, our live imaging system for C. elegans fertilization seems to be useful for evaluation of the functions of male and female gametes.
Description
Gamete fusion is a pivotal step during fertilization to create an organism of the next generation. In C. elegans, since oocytes have no thick egg coat like the zona pellucida, perhaps spermatozoa directly bind to and fuse with the oocyte plasma membrane (PM). Thus, C. elegans is an excellent model to investigate how a spermatozoon and an oocyte fuse together.
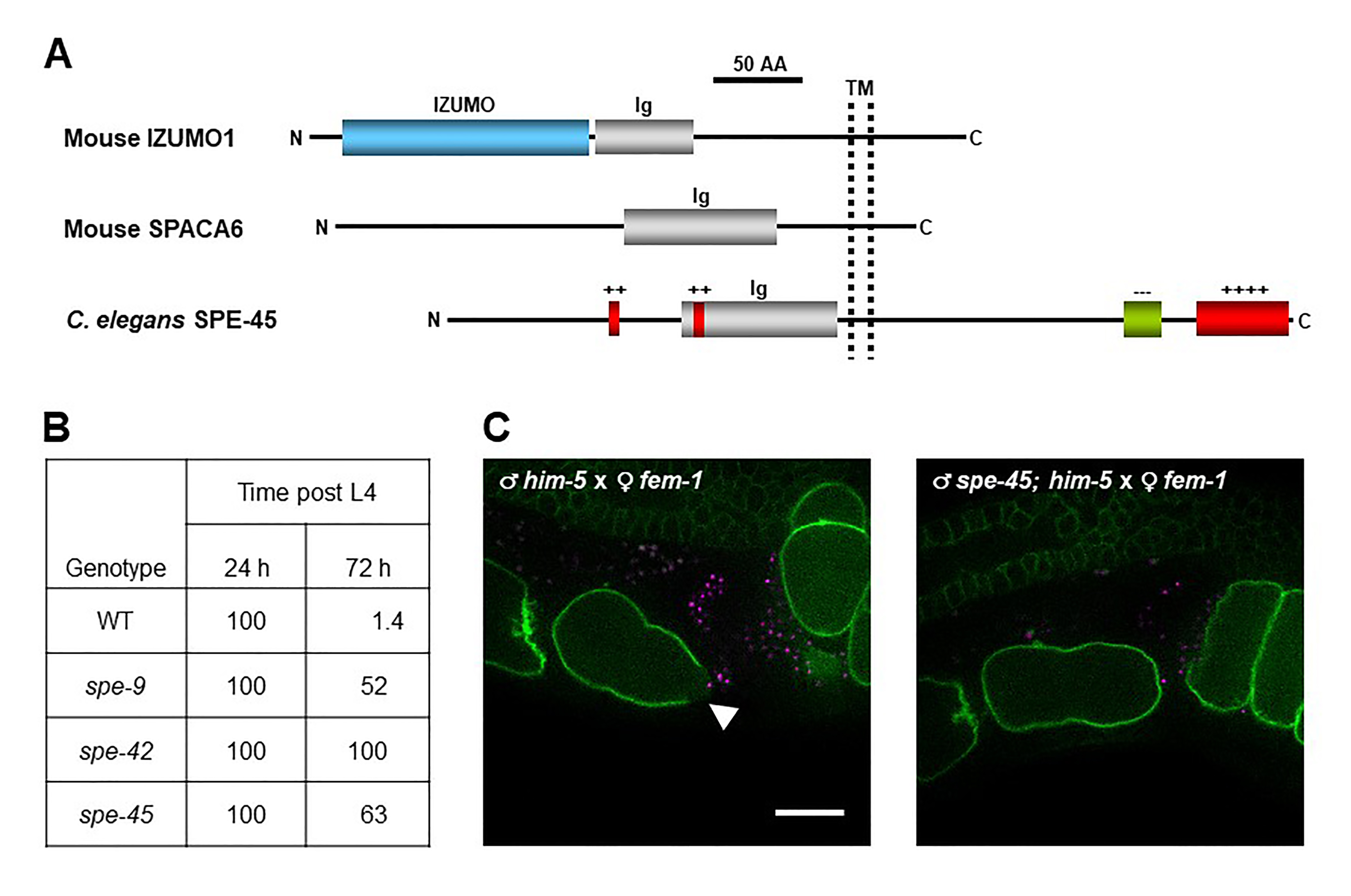
C. elegans spe genes encode proteins needed for male germline functions, such as spermatogenesis (meiosis to produce spermatids), spermiogenesis (transformation of spermatids into spermatozoa), and fertilization (gamete fusion) (L’Hernaut, 1997; Nishimura and L’Hernault, 2010; Singaravelu and Singson, 2011). In particular, spe-9 (Singson et al., 1998), spe-38 (Chatterjee et al., 2005; Singaravelu et al., 2012), spe-41/trp-3 (Xu and Sternberg, 2003; Singaravelu et al., 2012; Takayama and Onami, 2016), spe-42 (Kroft et al., 2005), and spe-49 (Wilson et al., 2018) belong to the spe-9 class, and these genes all play essential roles during fertilization. However, it has been unclear whether members of the spe-9 class have evolutionarily conserved roles during fertilization. To explore this question, we hypothesized that C. elegans has an ortholog of mouse Izumo1. This gene encodes a sperm transmembrane (TM) protein with a single immunoglobulin (Ig)-like domain that is required for sperm-oocyte fusion (Figure 1A) (Inoue et al., 2005; Inoue et al., 2015). Recently, mouse Spaca6 was also identified as a testis-enriched gene encoding an Ig-like TM protein with an indispensable role in gamete fusion, as is the case for mouse Izumo1 (Figure 1A) (Lorenzetti et al., 2014; Barbaux et al., 2020; Noda et al., 2020). Moreover, Ig-like proteins from various species are known to act in gamete interactions (Nishimura and L’Hernault, 2016). Thus, we searched for male germline-enriched genes encoding proteins with the Izumo1-like domain architecture in the C. elegans genome by reverse genetic approaches (Nishimura et al., 2015). spe-45 (formerly named F28D1.8/oig-7) meets these criteria (Figure 1A), and spe-45(tm3715) hermaphrodites exhibit typical Spe phenotypes; the mutant worms are self-sterile but fertile after outcrossing to wild-type (WT) males. Singaravelu et al. also identified the same gene by a forward genetic approach (Singaravelu et al., 2015).
Among the male germline functions, spermatogenesis and spermiogenesis are not impaired in spe-45(tm3715) worms (Singaravelu et al., 2015; Nishimura et al., 2015). As shown in Figure 1B, while self-sperm mostly disappeared in the spermatheca of WT hermaphrodites at 72 h post the fourth larval stage (L4), more than half of self-sperm still reside in the spermatheca of spe-9(eb19), spe-42(tm2421), and spe-45(tm3715) hermaphrodites even at the same time point (Nishimura et al., 2015). Thus, all the tested mutants are presumably incapable of completing fertilization (gamete fusion), indicating that spe-45 is a member of the spe-9 class. Additionally, a domain-swapping approach showed that the Ig-like domains of C. elegans SPE-45 and mouse IZUMO1 share a conserved role during fertilization despite only ~9% identity of the entire amino acid sequences of these two proteins (Nishimura et al., 2015).
The data in Figure 1B provide strong but indirect evidence that spe-45 is involved during gamete fusion. On the other hand, we have succeeded in live imaging of fertilization occurring in the spermatheca by two-color 3D time-lapse spinning-disc confocal microscopy (Takayama and Onami, 2016). In this system, we used spermatozoa from males carrying the oxIs318 allele, which produces mCherry-labeled sperm nuclei, and oocytes from ltIs38 hermaphrodites, of which the PM is tagged with a green fluorescent protein (GFP). Therefore, we produced him-5(e1490) and spe-45(tm3715); him-5(e1490) males in the oxIs318 background as WT-like and spe-45 mutant, respectively, and fem-1(hc17) hermaphrodites in the ltIs38 background as WT-like females.
After mating of fem-1 females with WT or spe-45 males, the spermatheca of the females was observed. From movies recording the live fertilization (see Extended Data), we could obtain still images showing a fluorescence gap on the oocyte PM, which was yielded by WT but not spe-45(tm3715) spermatozoa (Figure 1C). The place indicated by the fluorescence gap region seemed to represent where gamete fusion occurs, based on the previous report (Takayama and Onami, 2016). Therefore, our present findings show direct evidence that spe-45(tm3715) spermatozoa have a defect in sperm-oocyte fusion.
One of the advantages of using C. elegans for reproductive biology is that live fertilization in the spermatheca can be observed through transparent cuticles. Our imaging analysis demonstrates that spermatozoa lacking spe-45 fail to fuse with the oocyte PM, unlike WT spermatozoa. However, it remains to be clarified how each spe-9 class gene acts during gamete fusion. For instance, the data in Figure 1B imply that functional roles of spe-9 and spe-45 might be distinguishable from that of spe-42 during gamete fusion. Our imaging system would provide important clues to solve the point.
Methods
Request a detailed protocolWorm maintenance and culture
We maintained and cultured worms at 20ºC unless otherwise stated, according to standard methods (Brenner, 1974). him-5(e1490) was used as WT to produce males. spe-45(tm3715); him-5(e1490) males were obtained by picking GFP-negative males from the SL1491 strain. To generate males from strains without the him-5(e1490) background, we incubated L4 hermaphrodites at 32ºC for 5 h, and the resulting F1 males were picked to use for further experiments. As fem-1(hc17) worms were used as WT females, fertilized eggs of the strain were cultured at 25ºC until they reached the adult stage.
Microscopy
Most worm handling, such as picking and dissecting, were carried out under SZ61 or SZX10 dissecting microscopes (Olympus). To observe GFP signals on the oocyte PM for a screening of the HTN3 strain, we used an Olympus BX53 fluorescent microscope. For imaging experiments, a Nikon Ti-E microscope with the objective lens Plan Apo VC 60xA/1.20 W (Nikon) was used. Confocal microscopy was performed with the spinning-disk confocal unit CSU-X1 (Yokogawa Electric Corp.). The confocal unit was equipped with a solid-state laser lines (488 nm and 561 nm; ALC-501 AOTF, Andor Technology). Time-lapse images were acquired with an EM-CCD camera (iXon DU-897, Andor Technology). Scanning along the z-axis in 3D time-lapse imaging was performed using a piezo stage-positioning system (Nano-Drive, Mad City Labs). Settings for time-lapse imaging were as follows: 18 planes of 0.5 µm z-axis interval, 2 s time interval and 40 ms exposure for each color. The camera and the piezo system were synchronized using a precision controller unit (PCU-100, Andor Technology). Imaging systems were controlled using iQ software (Andor Technology).
Imaging of fertilization in the spermatheca
To obtain females, we raised fem-1(hc17); ltIs38 hermaphrodites at 25ºC. The feminized worms were mated with oxIs318; him-5(e1490) (WT) or oxIs318; spe-45(tm3715); him-5(e1490) (spe-45 mutant) males at 25ºC overnight (female:male = 1:4). Then, the females after mating were immobilized using polystyrene nanoparticles and agarose pads as previously described (Takayama and Onami, 2016). Fertilization occurring in the spermatheca of the immobilized worms was observed by two-color 3D time-lapse spinning-disc confocal microscopy.
Reagents
| Strain | Genotype | Source |
| DR466 | him-5(e1490) V | CGC |
| BA17 | fem-1(hc17ts) IV | CGC |
| SL1491 | spe-45(tm3715) IV; him-5(e1490) V; ebEx15-6[spe-45(+)(WRM066cF06) + myo-3p::gfp] | Dr. L’Hernault |
| OD58 | unc-119(ed3) III; ltIs38[pie-1p::gfp::PH(PLC1delta1) + unc-119(+)] | CGC |
| EG4883 | oxIs318[spe-11p::mCherry::H2B + unc-119(+)] II; unc-119(ed3) III | CGC |
| HTN3* | fem-1(hc17ts) IV; ltIs38[pie-1p::gfp::PH(PLC1delta1) + unc-119(+)] | This study |
| HTN4* | oxIs318[spe-11p::mCherry::H2B + unc-119(+)] II; him-5(e1490) V | This study |
| HTN5* | oxIs318[spe-11p::mCherry::H2B + unc-119(+)] II; spe-45(tm3715) IV; him-5(e1490) V;
ebEx15-6[spe-45(+)(WRM066cF06) + myo-3p::gfp] |
This study |
| *HTN3, HTN4 and HTN5 might contain the unc-119(ed3) allele, but genotype of unc-119 is not determined in these strains. | ||
| Primer | Nucleotide sequence | |
| HN63 | 5’-ACTCGAACCAATTGGCAGGT-3’ | |
| HN64 | 5’-CAAGGCGCACACTCTCTTTC-3’ | |
| NM3887 | 5’-ACCGGAAACCAAAGGACGAGAG-3’ | |
| NM3888 | 5’-ACGCCCAGGAGAACACGTTAG-3’ | |
To generate HTN3, we outcrossed BA17 hermaphrodites to OD58 males at 20ºC. F1 progeny were allowed to produce self-progeny. The F2 offspring were individually separated and allowed to produce F3 self-progeny. We individually picked five animals at the first larval stage (L1) among F3 progeny in each F2 plate, and the F3 larvae were cultured at 25ºC until they developed into adult worms. If all the five F3 worms from the same F2 plate became self-sterile, we judged that the F2 worm homozygously carries the fem-1(hc17) allele. In the F2 fem-1(hc17) worms, we also determined the genotype of ltIs38 by observing GFP-signals on the oocyte PM under an Olympus BX53 fluorescent microscope. A difference of GFP-signal intensities can be distinguishable between heterozygote and homozygote of ltIs38.
To generate HTN4, we outcrossed EG4883 hermaphrodites to DR466 males. F1 progeny were allowed to produce self-progeny. The F2 offspring were individually separated and allowed to produce F3 self-progeny. If a certain F2 plate contains sons as F3 worms, the genotype of oxIs318 in the F2 worm was determined by single-worm PCR (Nishimura et al., 2015), using a primer set of NM3887 and NM3889 (Frøkjær-Jensen et al., 2008).
To generate HTN5, we outcrossed EG4883 hermaphrodites to SL1491 males. GFP-positive F1 progeny were picked and then allowed to produce self-progeny. GFP-positive F2 offspring were individually separated and allowed to produce F3 self-progeny. If a certain F2 plate contains sons as F3 worms, five GFP-negative F3 males were picked from the plate to examine the genotypes of spe-45 and oxIs318 by single-worm PCR, using primer sets of HN63 and HN64 for spe-45 and NM3887 and NM3888 for oxIs318. If all the five tested F3 worms carried both spe-45(tm3715) and oxIs318 as homozygotes, GFP-positive hermaphrodites in the same F2 plate were maintained as oxIs318; spe-45(tm3715); him-5(e1490); ebEx15-6. As oxIs318; spe-45(tm3715); him-5(e1490) worms were required, GFP-negative animals were picked.
Extended Data:
Description: This is a movie file recording live fertilization between WT spermatozoa and WT oocytes.
Description: This is a movie file recording live fertilization between spe-45 mutant spermatozoa and WT oocytes.
Extended Data are archived at CaltechData.
Acknowledgments
We thank Dr. L’Hernault for providing the SL1491 strain and helpful comments on the manuscript. Other strains were provided from the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Extended Data
Nishimura, H. (2021). C. elegans spermatozoa lacking spe-45 are incapable of fusing with the oocyte plasma membrane: him-5 X fem-1.mov (Version 1.0). CaltechDATA. https://doi.org/10.22002/D1.1887 10.22002/D1.1887
Nishimura, H. (2021). C. elegans spermatozoa lacking spe-45 are incapable of fusing with the oocyte plasma membrane: spe-45;him-5 X fem-1.mov (Version 1.0). CaltechDATA. https://doi.org/10.22002/D1.1889 10.22002/D1.1889
References
Funding
This work was supported by MEXT KAKENHI Grant Number 24112716 (to H.N.) and JSPS KAKENHI Grant Number 24570241 (to H.N.).
Reviewed By
Andrew SingsonHistory
Received: February 17, 2021Accepted: February 18, 2021
Published: February 21, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Takayama, J; Tajima, T; Onami, S; Nishimura, H (2021). C. elegans spermatozoa lacking spe-45 are incapable of fusing with the oocyte plasma membrane. microPublication Biology. 10.17912/micropub.biology.000372.Download: RIS BibTeX