Abstract
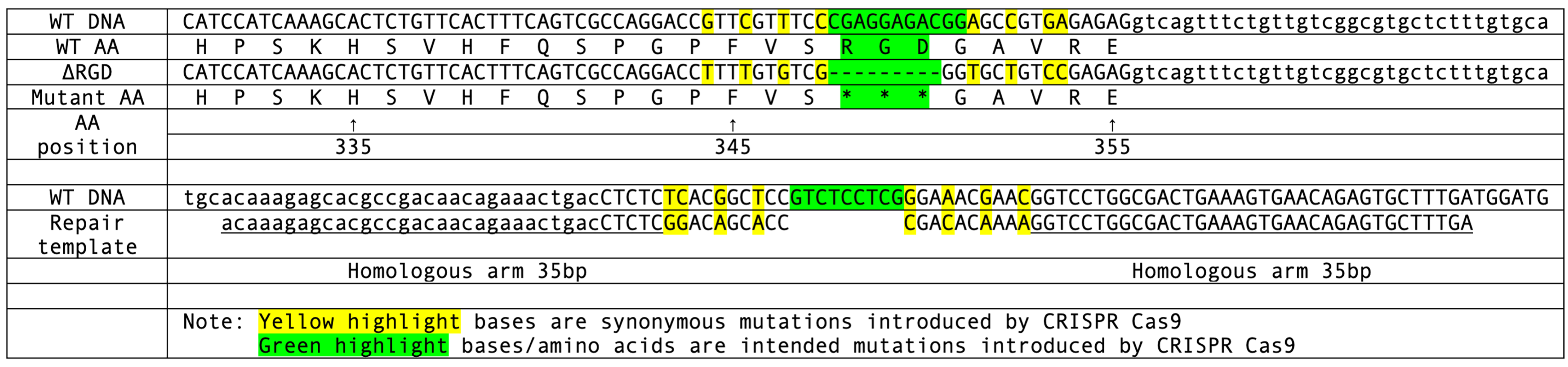
The lon-2 gene in Caenorhabditis elegans encodes a heparan sulfate proteoglycan family glypican that negatively regulates the BMP signaling pathway responsible for controlling body length. LON-2 contains multiple functional domains, including an RGD (Arg-Gly-Asp) motif at amino acid number from 348 to 350. A novel mutant allele of lon-2 was investigated in this study. In this mutant allele, lon-2(kq348ΔRGD), the RGD motif at position 348 was deleted. Another pre-existing mutant allele, lon-2(e678), contains a ~9kb deletion and lacks most of the genomic coding sequence. The lon-2(e678) line was used as a reference allele. The novel mutant line was significantly shorter than wild-type animals, suggesting that removal of the RGD motif in LON-2 may improve its ability to inhibit BMP signaling.
Description
The lon-2 gene in Caenorhabditis elegans encodes a heparan sulfate proteoglycan family glypican that negatively regulates the BMP signaling pathway responsible for controlling body length (Gumienny et al., 2007). LON-2 contains multiple functional domains, including an RGD (Arg-Gly-Asp) motif at amino acid number from 348 to 350. RGD motifs are the cell-binding site of the extracellular matrix protein and are known to interact with cell surface receptors of the extracellular matrix, integrins (Margadant and Sonnenberg, 2010). Previous studies show that this RGD motif in LON-2 does not directly regulate BMP, but is essential to the function of soluble LON-2, suggesting that the RGD motif likely utilizes another part of the LON-2 N-terminal core glypican protein functional domain to play a role in BMP signal regulation (Taneja-Bageshwar and Gumienny 2012). These studies indicated that LON-2 acts by binding and sequestering DBL-1 and acting as an RGD-binding protein, modulating the strength of the BMP signal and thereby negatively regulating body size (Gumienny et al., 2007; Taneja-Bageshwar and Gumienny 2012). Previous analysis demonstrated that lon-2(e678) mutants are unable to regulate DBL-1 signaling and as a result of higher doses of DBL-1 are longer than wild-type animals (Gumienny et al., 2007; Suzuki et al., 1999). A novel mutant allele of lon-2 was investigated in this study. In this mutant allele, lon-2(kq348ΔRGD), the RGD motif at position 348 was deleted. The previously mentioned pre-existing mutant allele, lon-2(e678), contains a ~9kb deletion and lacks most of the genomic coding sequence (Gumienny et al., 2007). The lon-2(e678) line was used as a reference allele in order to identify the presence of morphological abnormalities (the long phenotype) in our novel mutant line. RGD mutant worms as well as wild-type worms were imaged and measured in order to screen for the presence or absence of the long phenotype in the novel mutant line. 45 wild-type animals (n=45) and 27 lon-2(ΔRGD) animals (n=27) were imaged and measured in the assay. Wild-type animals had an average body length of 1127.6 µm (SE ± 25.28 µm) and the mutant line had an average body length of 968.6 µm (SE ± 22.98 µm). The 99% confidence intervals for body size of the two lines did not overlap, indicating a statistically significant difference in body length. This suggests that deletion of the RGD motif at position 348 in LON-2 may improve its ability to inhibit BMP signaling, resulting in a shorter body length. The mechanism by which this occurs remains a topic for future investigation, but may happen due to the lack of normal interactions of the RGD motif with integrins and other proteins. Recent research revealing interactions between LON-2 and LIN-17/Frizzled (Saied-Santiago et al.., 2017) could guide future research toward investigating the relationship between the LON-2 RGD domain and Frizzled/Wnt signaling.
Methods
Request a detailed protocolCRISPR target sites were identified using the CRISPR guide RNA Selection Tool (http://genome.sfu.ca/crispr/) in order to ensure that the desired mutations would be created in lon-2. The first mutant line lon-2(ΔRGD) contains a total deletion of the RGD motif at amino acid position 348 (Figure A). The second mutant line lon-2(e678) is a reference allele that has not yet been sequenced, but contains a large ~9kb deletion that removes significant portions of the genomic coding sequence in lon-2 (Gumienny et al., 2007). The syncytial gonad arms of N2 wild-type worms (P0) were micro-injected with a mixture containing custom template DNA (Temp-4LON2ΔRGD, Figure A), custom crRNA (LON2RGD350), tracrRNA (cat. #1072532), and Alt-R Cas9 nuclease (cat. #1081058) that had been annealed at room temperature (Mello et al., 1991; Paix et al.., 2015). Primers, repair template, sgRNA and Cas9 enzymes were purchased from IDTDNA Inc., Coralville, IA. F1 worms were then isolated and PCR screened using the mutant specific primer (LON2ΔRGD350F) to ensure that the desired mutation had been induced. Both wild-type (LON2RGD350WTF) and mutant specific (LON2ΔRGD350F) primers were then used in PCR screening of the F2 offspring in order to isolate homozygous mutants. The PCR products were then sequenced to confirm that the mutant allele had been successfully created (Psomagen Inc, Rockville, MD). Both mutant lines were backcrossed (2 times) to N2 and the novel RGD mutant line was then studied to characterize phenotype. 45 wild-type and 27 lon-2(ΔRGD) animals were imaged and measured to screen for the presence of the long phenotype. Worms were first paralyzed using 10 mM levamisole and were then examined under Nomarski optics using a Nikon NiU epifluorescence microscope (Nikon, Melville, NY). The Nikon Element software was then used to digitally measure the body lengths. Results from this assay were then analyzed using a Mann-Whitney U Test in order to obtain quantitative measures of statistical significance.
crRNA sequence
LON2RGD350: ACAGAAACTGACCTCTCTCA
PCR primers
LON2RGD350WTF: CCGAGGAGACGGAGCCGTGA
LON2ΔRGD350F: CTTTTGTGTCGGGTGCTGTCC
LON2RGD350SEQF: CCGACCCCTTTCCTCATGATT
LON2RGD350SEQR: TCAAATCCGCCAAATCAGGCT
Reagents
lon-2(kq348), referred to as lon-2(ΔRGD), is available upon request.
Acknowledgments
This study was conducted during the course of BIO 3V90 at Baylor University.
References
Funding
Funding for BIO 3V90 is provided by Baylor University
Reviewed By
Tina GumiennyHistory
Received: February 9, 2021Revision received: March 3, 2021
Accepted: March 8, 2021
Published: March 10, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Park, A; Qiu, Z; Lee, M (2021). Deletion of the RGD motif in LON-2/glypican is associated with morphological abnormalities. microPublication Biology. 10.17912/micropub.biology.000376.Download: RIS BibTeX