Department of Genetics, College of Agricultural and Life Sciences, University of Wisconsin-Madison, Madison, WI 53706
Abstract
Traumatic brain injury (TBI) frequently leads to non-neurological consequences such as intestinal permeability. The beta-blocker drug labetalol, which inhibits binding of catecholamine neurotransmitters to adrenergic receptors, reduces intestinal permeability in a rat TBI model. Using a Drosophila melanogaster TBI model, we previously found a strong positive correlation between intestinal permeability and mortality within 24 hours of TBI in a standard laboratory line (w1118) and across genetically diverse inbred lines from the Drosophila Genetic Reference Panel (DGRP). Here, we report that feeding injured w1118 flies the beta-blockers labetalol and metoprolol reduced intestinal permeability and mortality. Additionally, metoprolol reduced intestinal permeability when 18 DGRP fly lines were analyzed in aggregate, but neither beta-blocker affected mortality. These data indicate that the mechanism underlying disruption of the intestinal barrier by adrenergic signaling following TBI is conserved between humans and flies and that mortality following TBI in flies is not strictly dependent on disruption of the intestinal barrier. Thus, the fly TBI model is useful for shedding light on the mechanism and consequences of adrenergic signaling between the brain and intestine following TBI in humans.
Description
Traumatic brain injury (TBI) is a substantial public health problem with treatment made difficult by unique neurological sequelae of individual cases (Johnson and Griswold 2017; Pavlovic et al. 2019). Pathological processes evolve over time after TBI and are associated with complex changes in neurotransmitter systems (McGuire et al. 2019). Relevant neurotransmitters include catecholamines such as norepinephrine, epinephrine, and dopamine that target adrenergic receptors (Jenkins et al. 2016). Elevated levels of circulating catecholamines in plasma, in particular epinephrine, at the time of hospital admission after TBI are associated with increased risk of worse functional outcomes and mortality (Woolf et al. 1987; Rizoli et al. 2017). Furthermore, retrospective and prospective studies show that TBI patients treated with beta-blockers, agents that block binding of epinephrine to its receptor, have a significantly reduced risk of mortality (Cotton et al. 2007; Schroeppel et al. 2010; Alali et al. 2014; Mohseni et al. 2015; Ko et al. 2016; Khalini et al. 2020; Florez-Perdomo et al. 2021). Beta-blockers appear to act on trauma-induced signals from the brain, since non-head trauma patients treated with beta-blockers do not have a reduced risk of mortality (Hendrick et al. 2016).
Beta-blockers may elicit beneficial effects in TBI by reducing intestinal permeability, as indicated by a study of the beta-blocker labetalol in a rat TBI model (Lang et al. 2015). In mammals, bidirectional signaling between the brain and intestine, more generally known as the brain-gut axis, plays a significant role in TBI (Pimentel et al. 2012; Al Omran and Aziz 2014; Katzenberger et al. 2015c; Mittal et al. 2017; Weaver et al. 2021). Direct mechanical damage to the brain in rodent TBI models causes disruption of the intestinal barrier, and in the first few weeks after injury, TBI patients frequently have reduced intestinal contractile activity and absorption that can lead to intestinal permeability (Faries et al. 1998; Hang et al. 2003; Feighery et al. 2008; Jin et al. 2008; Bansal et al. 2009; Bansal et al. 2010; Ma et al. 2017; Pan et al. 2019). Despite evidence that beta-blockers attenuate functional deficits after TBI, more research is needed to understand the underlying mechanisms as well as potentially confounding effects of diverse genetic and environmental factors (Heffernan et al. 2010; Osier et al. 2016).
To study the effect of genetic and environmental factors on TBI outcomes, we developed a Drosophila melanogaster model of closed-head TBI (Katzenberger et al. 2013, 2015b). The fly model uses a spring-based, High-Impact Trauma (HIT) device to inflict TBI. Injuries inflicted by the HIT device lead to intestinal permeability and early mortality, suggesting that secondary injury mechanisms are conserved between humans and flies (Katzenberger et al. 2013, 2015a, 2016). Our measure of early mortality is the Mortality Index at 24 h (MI24), which is the percent mortality of injured flies minus the percent mortality of uninjured flies within 24 h. The MI24 is affected by diet following TBI. For example, the MI24 is lower for flies fed water versus high-carbohydrate diets following TBI (Katzenberger et al. 2015a, 2016). Our measure of intestinal permeability is the Smurfing Index at 24 h (SI24), which is the percent of injured flies that smurf minus the percent of uninjured flies that smurf within 24 h. In the Smurf assay, flies are fed a nonabsorbable blue dye prior to the injury. If the intestinal barrier is intact following the injury, the dye remains in the digestive tract, but if the intestinal barrier is disrupted, the dye crosses the barrier into the circulatory fluid (i.e., hemolymph) and disperses throughout the body in a process referred to as ‘smurfing’ because it results in a blue body akin to Smurf cartoon characters (Rera et al. 2012; Martins et al. 2018). The HIT device does not deliver head-specific injuries, but a crushing injury to the head is sufficient to cause flies to smurf, suggesting that intestinal barrier dysfunction following injuries from the HIT device is due to brain injuries (Katzenberger et al. 2015a).
Our prior analyses of genetically diverse inbred fly lines from the Drosophila Genetic Reference Panel (DGRP) revealed that the SI24 shows near perfect correlation with the MI24, that is, almost every fly that smurfs dies within 24 h, whereas very few flies that do not smurf die within 24 h (Katzenberger et al. 2015a). These data suggest that intestinal permeability is closely associated with early mortality in the fly TBI model. Disruption of the intestinal barrier and early mortality via adrenergic signaling is a possibility in the fly TBI model as well because flies synthesize the catecholamines tyramine and octopamine, which are structurally similar to epinephrine (Roeder, 2005), and signaling through tyramine and octopamine receptors modulates brain-wide states such as arousal as well as behaviors such as aggression (Hardie et al. 2007; Zhou et al. 2008; Busch et al. 2009; Andrews et al. 2014; Watanabe et al. 2017).
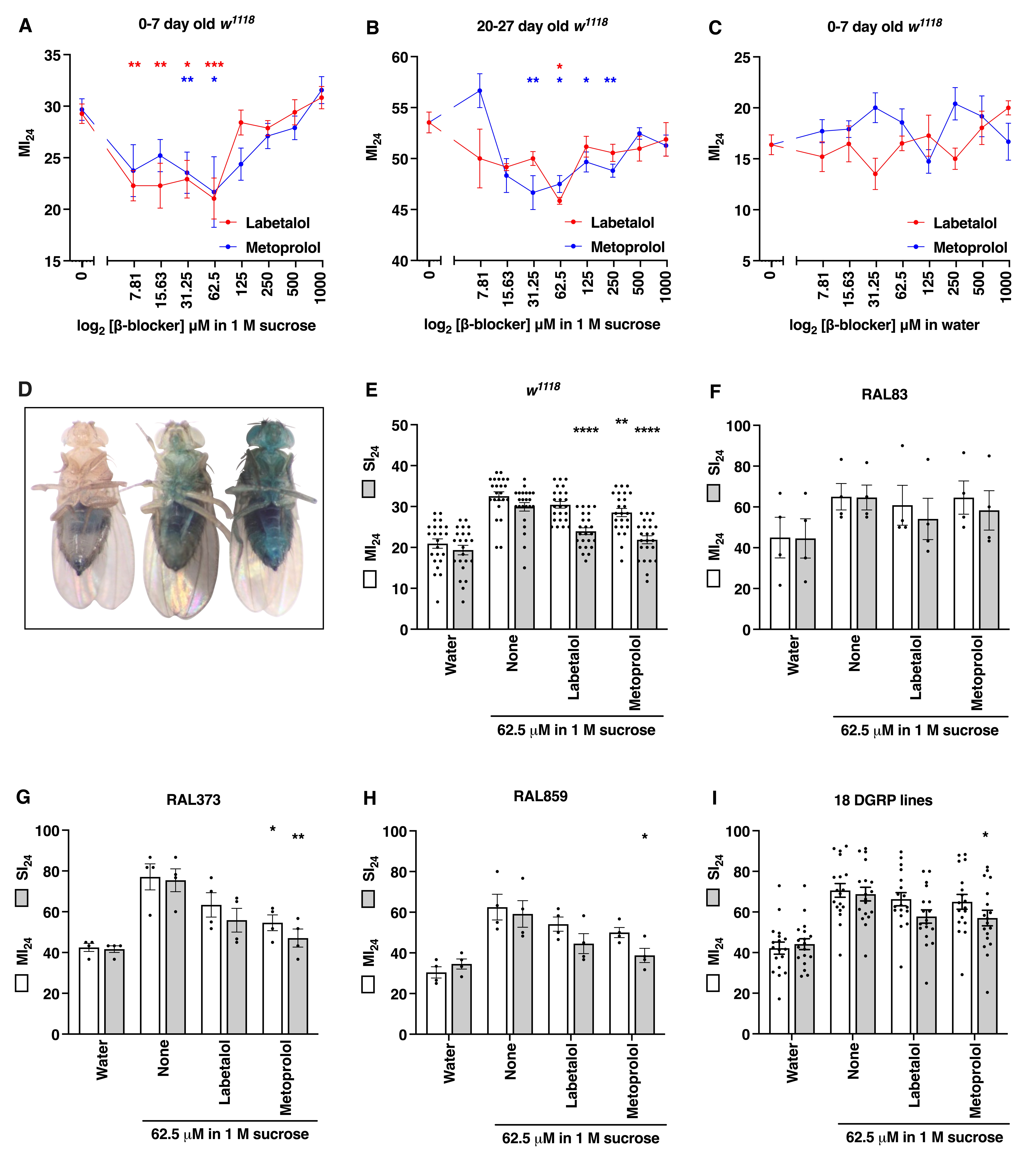
To investigate potential roles of adrenergic signaling in early mortality following TBI, we fed 0-7 day old w1118 flies the beta-blocker labetalol or metoprolol at concentrations ranging from 7.81 μM to 1000 μM in 1 M sucrose over the 24 h following TBI and measured the MI24. At 62.5 μM, both beta-blockers caused a significant reduction in the MI24 (p<0.05) (Fig. 1A). A similar beneficial effect of beta-blockers was observed for 20-27 day old w1118 flies that had a higher MI24 (p<0.05) (Fig. 1B). As is the case for many other compounds tested for efficacy in mammalian TBI models, both labetalol and metoprolol showed U-shaped dose-responses, indicating that too much or too little adrenergic signaling enhances early mortality following TBI (Calabrese et al. 2008). In contrast, none of the beta-blocker concentrations in water significantly reduced the MI24 when fed to 0-7 day old w1118 flies that had a lower MI24 (Fig. 1C). Taken together, these data indicate that adrenergic signaling triggers secondary injuries that promote mortality following TBI in both younger and older flies. Furthermore, different effects of beta-blockers delivered in sucrose versus water suggest that adrenergic signaling enhances carbohydrate-mediated secondary injuries.
To investigate whether adrenergic signaling mediates intestinal permeability following TBI and if so, whether modification of intestinal permeability affects early mortality, we fed 0-7 day old w1118 flies labetalol or metoprolol at 62.5 μM in 1 M sucrose over the 24 h following TBI and measured the SI24 and MI24. Figure 1D shows the range of blue body coloration that was scored as positive for smurfing. Both beta-blockers significantly reduced the SI24 (p<0.001), but in contrast to data in panel A, only metoprolol reduced the MI24 (p<0.01) (Fig. 1E). Additionally, w1118 flies fed water had similar SI24 and MI24 values that were substantially lower than those of w1118 flies fed 1 M sucrose, demonstrating that even when the SI24 and MI24 are reduced they remain similar when adrenergic signaling is intact. We repeated the experiment with 18 randomly selected lines from the DGRP. Representative data for three lines (RAL83, RAL373, and RAL859) are shown in panels F-H, and collective data for the 18 DGRP lines are shown in panel I. Neither beta-blocker affected the SI24 or MI24 of RAL83 flies, but metoprolol significantly reduced the SI24 (p<0.01) and the MI24 (p<0.05) of RAL373 flies and metoprolol significantly reduced the SI24 (p<0.05) of RAL859 flies (Figs. 1F-H). Analysis of the 18 DGRP lines in aggregate showed that metoprolol significantly reduced the SI24 (p<0.05), but neither beta-blocker affected the MI24 (Fig. 1I). Stronger effects of beta-blockers in DGRP fly lines may have been observed if beta-blocker doses were optimized for each line, as they were for w1118 flies. Even so, among flies of different genotype, beta-blockers reduced intestinal permeability to a greater extent than early mortality.
These data indicate that following TBI in flies, adrenergic signaling mediates intestinal permeability and early mortality. Furthermore, since beta-blockers reduced intestinal permeability without affecting early mortality in some fly lines, early mortality is not strictly dependent upon intestinal permeability. However, the disconnect between intestinal permeability and early mortality may be due to the inability of the Smurf assay to detect low amounts of intestinal permeability that could be sufficient to cause mortality. Nevertheless, this study demonstrates that in flies, as in mammals, adrenergic signaling triggers intestinal permeability following TBI. As a result, the fly TBI model can be used to investigate the mechanism underlying TBI-induced adrenergic signaling between the brain and intestine and the influence of genetic and environmental factors on the mechanism.
Methods
Request a detailed protocolFly lines and culturing
Flies were maintained in humidified incubators at 25°C in vials containing cornmeal molasses food (Katzenberger et al. 2015). DGRP lines were obtained from the Bloomington Stock Center, and w1118 flies were obtained from Dr. Gerald Rubin’s lab (University of California-Berkeley) and maintained for 25 years. DGRP lines used in the study included RAL26 (DGRP-26/FBsn0000007), RAL83 (DGRP-83/FBsn0000021), RAL161 (DGRP-161/FBsn0000036), RAL332 (DGRP-332/FBsn0000072), RAL352 (DGRP-352/FBsn0000079), RAL373 (DGRP-373/FBsn0000091), RAL374 (DGRP-374/FBsn0000092), RAL382 (DGRP-382/FBsn0000099), RAL391 (DGRP-391/FBsn0000104), RAL441 (DGRP-441/FBsn0000117), RAL491 (DGRP-491/FBsn0000122), RAL555 (DGRP-555/FBsn0000134), RAL707 (DGRP-707/FBsn0000146), RAL761 (DGRP-761/FBsn0000159), RAL774 (DGRP-774/FBsn0000162), RAL818 (DGRP-818/FBsn0000177), RAL859 (DGRP-859/FBsn0000191), and RAL907 (DGRP-907/FBsn0000202).
Treatment with beta-blockers
Stock solutions of 1 mM labetalol and metoprolol (Sigma, St. Louis, MO) were prepared in 1 M sucrose (Sigma) or water and serially diluted 2-fold in 1 M sucrose or water to 7.81 μM. Solutions of labetalol and metoprolol as well as 1 M sucrose and water were fed to flies by placing 200 μl on a filter paper disc at the bottom of a vial.
MI24 and SI24 assays
MI24 values were determined as described in Katzenberger et al. 2013 and 2015b. SI24 values were determined as described in Katzenberger et al. 2015a, based on the Smurf assay described in Rera et al. 2012 and Martins et al. 2018. In panels A-C and E, TBI was inflicted by four strikes from HIT device #1 with 5 min between strikes, and in panels D and F-I, TBI was inflicted by three strikes from HIT device #9 with 5 min between strikes. In each biological replicate, a vial contained 60 flies (approximately 30 males and 30 females).
Acknowledgments
We thank the Boekhoff-Falk, Ganetzky, Perouansky, and Wassarman labs for contributions to this work. We also thank Jaret Schroeder for the idea of studying the role of adrenergic signaling in the fly TBI model and Douglas Wassarman for assistance with data presentation.
References
Funding
This work was supported by NIH grant RF1 NS114359 to BG and DAW. ARS was supported by a Women in Science & Engineering (WISE) Summer Research Award, a Genetics Department Summer Fellowship, and a Sophomore Research Fellowship from UW-Madison. MCF was supported by a UW-Madison Genetics Department Summer Fellowship.
Reviewed By
AnonymousHistory
Received: July 12, 2021Revision received: September 20, 2021
Accepted: September 20, 2021
Published: October 1, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Scharenbrock, AR; Katzenberger, RJ; Fischer, MC; Ganetzky, B; Wassarman, DA (2021). Beta-blockers reduce intestinal permeability and early mortality following traumatic brain injury in Drosophila. microPublication Biology. 10.17912/micropub.biology.000461.Download: RIS BibTeX