Abstract
Reproductive adults and developmentally arrested larvae often occupy different ecological niches and thus are expected to respond differently to environmental stimuli. To understand the genes that coordinate dauer development and olfactory behavior, we examined adult and dauer C. elegans in wild-type and dauer constitutive mutants (Daf-c). We found all dauers showed decreased attraction to all three odorants tested compared to adults, with daf-7 dauer larva (DL) exhibiting a concentration-dependent preference shift towards isoamyl alcohol, suggesting that the TGF-ß pathway is involved in both dauer regulation and dauer-specific odortaxis.
Description
In C. elegans, the dauer larva (DL) is a non-feeding and stress-resistant stage that can respond to environmental cues differently from adults. For example, C. elegans adults avoid CO2 while DL are attracted to CO2 (Hallem and Sternberg 2008; Hallem et al. 2011). Similarly, adults of the nematode Pristionchus pacificus are mildly attracted to a beetle host pheromone while its dispersive DL are highly attracted to the pheromone (Carstensen et al. 2021).
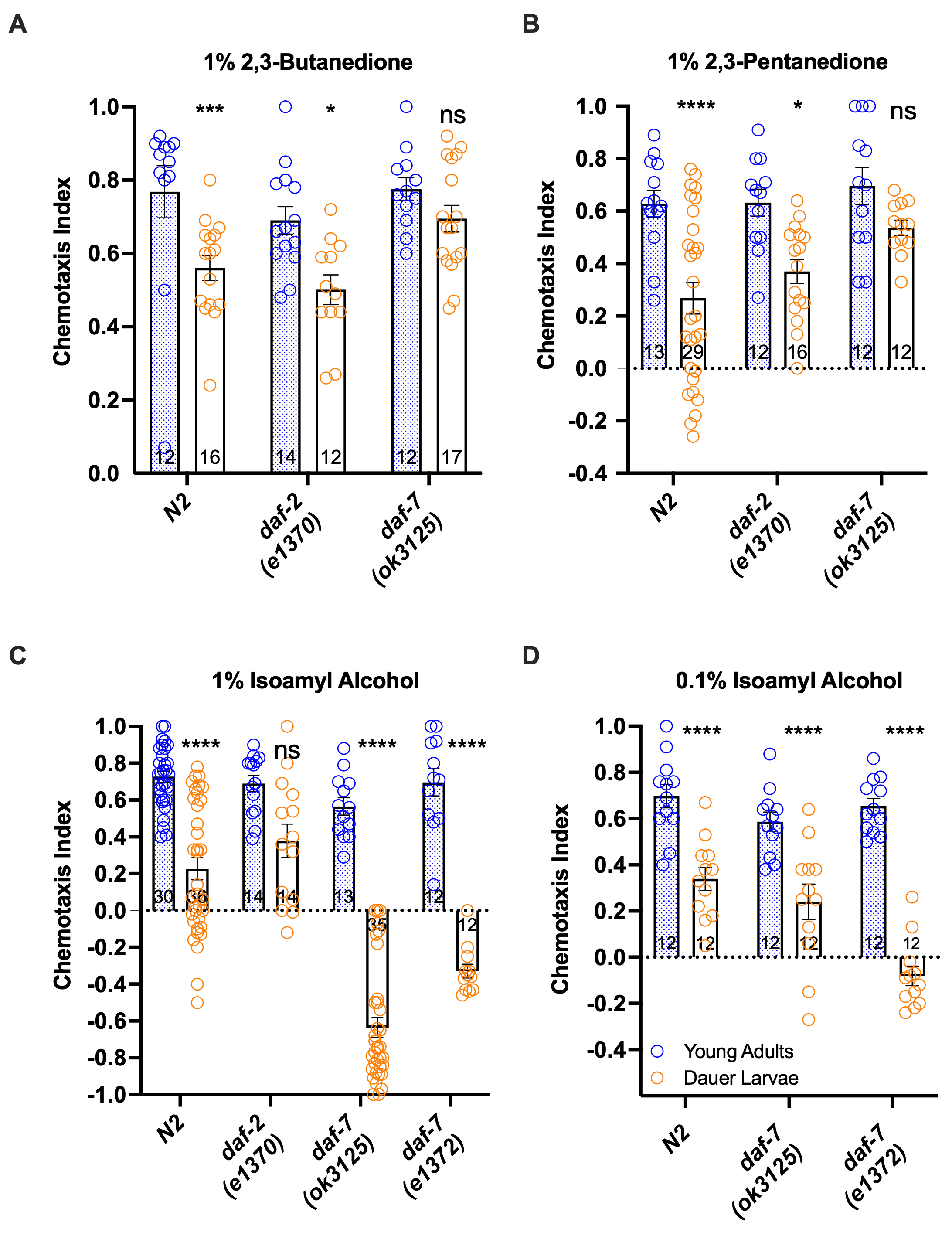
To determine if genes that affect dauer development also modulate olfactory behavior in C. elegans, we compared the odor response profiles of young adults versus DL in C. elegans of two well-studied Daf-c mutants, daf-2 and daf-7 (Riddle et al. 1981; Swanson and Riddle 1981; Kimura et al. 1997) towards three odorants. In C. elegans adults, the AWA neurons detect 2,3-butanedione (diacetyl), while the AWC neurons sense isoamyl alcohol (IAA) and 2,3-pentanedione (Sengupta et al. 1996; Chalasani et al. 2007). We found that wild-type DL showed less odor attraction than corresponding adults for 2,3-butanedione, 2,3-pentanedione, and IAA, consistent with the results of a previous study that included 2,3-butanedione (Hallem et al. 2011)(Fig. 1A-C). However, this adult-DL difference was not observed in daf-2 towards IAA or daf-7 towards 2,3-butanedione and 2,3-pentanedione. Furthermore, the response to IAA changed from attractive to repulsive in the DL of two daf-7 alleles (e1372, ok3125). To evaluate if the avoidance response to IAA is due to hypersensitivity to IAA in the daf-7 DL, we also tested their response to a 10-fold lower IAA concentration (0.1%). We found that the strong avoidance response was no longer observed, and instead the dauer IAA attraction became significantly reduced in both daf-7 alleles compared to adults, resembling the wildtype DL (Fig. 1D). In C. elegans, odor concentration-dependent preference shift has been observed for IAA for adult worms and is dependent on odr-3 function (Yoshida et al. 2012). Thus, C. elegans wild-type and Daf-c DL showed decreased attraction compared to adults to all three odorants tested, with the daf-7 mutations producing an accentuated concentration-dependent response to IAA in the DL, suggesting that the TGF-ß pathway is involved in both dauer regulation and dauer-specific response to IAA.
While remodeling of the AWC dendritic ends that increase their surface area have been speculated to heighten odor sensitivity in DL (Albert and Riddle 1988), our results show that only daf-7 alleles exhibited a potentially higher sensitivity to isoamyl alcohol, while wild-type and daf-2 DL were actually less attracted than adults to several odors. Mutation of the daf-2 gene also mostly eliminated acute CO2 avoidance in adult C. elegans, while the P. pacificus Daf-c mutant Ppa-hsd-2 exhibited greatly enhanced adult attraction to a host odor (Hallem and Sternberg 2008; Carstensen et al. 2021). These results suggest that dauer development has multiple effects on olfactory behavior, such that both wild-type and Daf-c DL should be assessed when surveying species-specific responses.
Methods
Request a detailed protocolNematodes were cultured on OP50-seeded NGM plates and assayed at ~22°C. The chemotaxis assays for C. elegans were set up in a quadrant with worms loaded onto the center of 6 cm plates containing MOPS/Tween agar, along with two opposing pairs of odors and controls (Nuttley et al. 2002; Margie et al. 2013; Carstensen et al. 2021). 0.5 µl of 1 M sodium azide was spotted onto each odor or counter-attractant. Since C. elegans DL are hydrophilic, we collected DL primarily from the condensations present on the underside of the lids of recently starved plates by washing the lids with sterile spring water (Arrowhead, CA). For assays with Daf-c mutants, each plate was scored for either DL or YA using visual confirmation. Chemotaxis assays lasted 1-1.5 hours.
| Strain | Genotype |
| CB1370 | daf-2(e1370)III |
| CB1372 | daf-7(e1372)III |
| RB2302 | daf-7(ok3125)III |
| N2 | wildtype |
Reagents
MOPS buffer (pH 7)
Tween 20 (Polyoxyethylene 20-Sorbitan Monolaurate) CAS 9005-64-5
2,3-butanedione, isoamyl alcohol, and 2,3-pentanedione (Sigma-Aldrich)
References
Funding
NIH TL4GM118977 to JV; NIH GM105579 to RLH
Reviewed By
Ian Chin-SangHistory
Received: September 8, 2021Revision received: September 22, 2021
Accepted: September 22, 2021
Published: October 14, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Vertiz, J; Carstensen, H; Hong, R (2021). Dauer Development Modulates Olfactory Behavior. microPublication Biology. 10.17912/micropub.biology.000479.Download: RIS BibTeX