The Pennsylvania State University
Abstract
Nicotinamide recycling is critical to the development and function of Caenorhabditis elegans. Excess nicotinamide in a pnc-1 nicotinamidase mutant causes the necrosis of uv1 and OLQ cells and a highly penetrant egg laying defect. An EGF receptor (let-23) gain-of-function mutation suppresses the Egl phenotype in pnc-1 animals. However, gain-of-function mutations in either of the known downstream mediators, let-60/ Ras or itr-1, are not sufficient. Phosphatidylcholine synthesis is neither required nor sufficient, in contrast to its role in the let-23gf rescue of uv1 necrosis. The mechanism behind the let-23gf suppression of the pnc-1 Egl phenotype is unknown.
Description
NAD+ is an electron carrier and a co-substrate for NAD+-dependent enzymes such as poly(ADP-ribose) polymerases (Bouchard et al. 2003; Sauve 2008). The byproduct of these enzymatic reactions, nicotinamide (NAM), must be salvaged to maintain a readily available NAD+ pool. In Caenorhabditis elegans the nicotinamidase PNC-1 acts both cell autonomously and non-cell autonomously to convert NAM into nicotinic acid (NA), an NAD+ precursor in this organism (Huang and Hanna-Rose 2006; Vrablik et al. 2009; Crook et al. 2014). Loss of PNC-1 function affects NAD+ pathway metabolites in two ways. It results in an increase in NAM, causing necrosis of OLQ and uv1 cells, and an egg-laying phenotype due to reduced muscle function. It also reduces NAD+ levels, resulting in gonad developmental delay and a male mating defect (Huang and Hanna-Rose 2006; Vrablik et al. 2009; Vrablik et al. 2011; Upadhyay et al. 2016).
LET-23 is the sole C. elegans Epidermal Growth Factor (EGF) receptor and is involved in a range of biological and developmental processes, including vulval development and specification of the uv1 cells (Chang et al. 1999; Moghal and Sternberg 2003). A gain-of-function mutation in the extracellular domain, let-23(sa62)gf, results in precocious activation of LET-23 independent of its EGF ligand LIN-3 (Katz et al. 1996). Overactivation of LET-23 rescues the uv1 cell necrosis phenotype of pnc-1 loss-of-function mutants, and this rescue requires phosphatidylcholine synthesis (Huang and Hanna-Rose 2006; Crook et al. 2016; Crook and Hanna-Rose 2020). We noted that the egg-laying phenotype of pnc-1 was also ameliorated by overactivation of LET-23 and decided to investigate the mechanism.
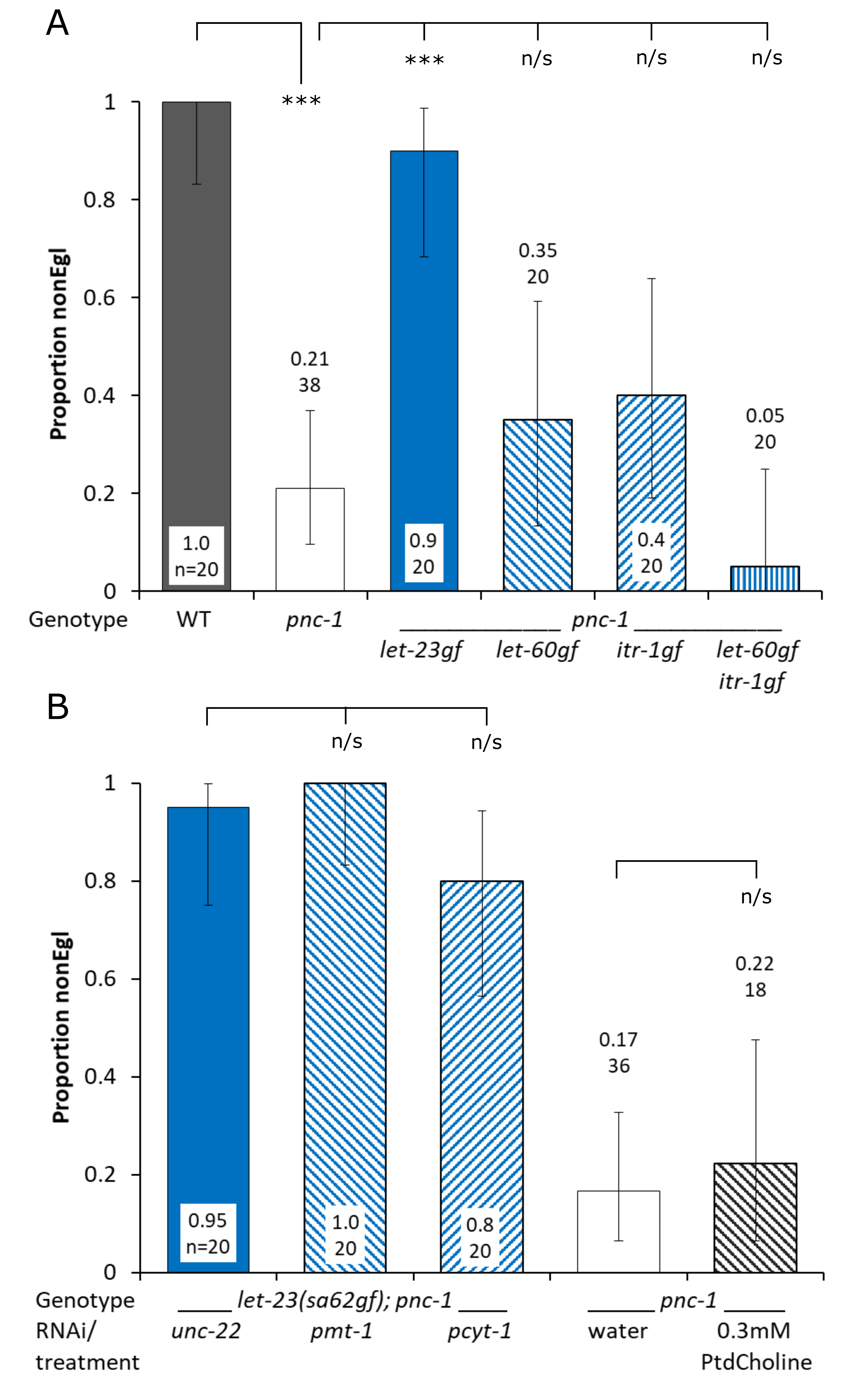
To study the role of EGF signaling in the prevention of the egg-laying phenotype we placed individual L4 hermaphrodites on Nematode Growth Medium (NGM) agar plates spotted with Escherichia coli OP50. Individual animals were observed after two, three and four days at 20C and scored as non-Egg laying defective (nonEgl) adults or “bags of worms” (Egl), where larvae hatch in the uterus due to a failure to lay eggs. Proportion nonEgl was calculated as the number nonEgl adults/ total number of individuals at day four. All nonEgl adults had laid eggs by day 4. We found that the pnc-1(pk9605) loss-of-function allele reduced the proportion of nonEgl adults to 0.21 and that the let-23(sa62) gain-of-function (gf) allele in a pnc-1(pk9605) background restored that to 0.9 (Fig. 1a). However, gain-of-function mutations in let-60 or itr-1, which mediate signal transduction downstream of let-23 (Clandinin et al. 1998; Chang et al. 1999), had no effect on the pnc-1 egg-laying phenotype (Fig. 1a).
Phosphatidylcholine synthesis is required for let-23(sa62)gf mediated rescue of uv1 necrosis and exogenous phosphatidylcholine alone is partially sufficient for uv1 survival (Crook et al. 2016). PMT-1 is part of the Sequential Methylation Pathway (SMP) that synthesizes phosphocholine (Brendza et al. 2007), and PCYT-1 turns phosphocholine from the Sequential Methylation and Kennedy pathways into CDP-choline, the precursor of phosphatidylcholine (Kennedy and Weiss 1956). To test if phosphatidylcholine synthesis was required for the let-23(sa62)gf-mediated rescue of the pnc-1 egg-laying phenotype we knocked down pmt-1 or pcyt-1 by RNAi. unc-22 (control), pmt-1 and pcyt-1 RNAi bacterial cultures were spotted onto NGM plates containing 50 μg.ml-1 ampicillin and 1 mM IPTG, then individual L4 hermaphrodites were added to each plate and scored as above. We found that neither pmt-1 nor pcyt-1 were required for rescue in a let-23(sa62)gf; pnc-1(pk9605) background (Fig. 1b). pmt-1 or pcyt-1 RNAi did however reduce uv1 cell survival in nonEgl adults in experiments run concurrently with this project (Crook et al. 2016), suggesting that RNAi knockdown of the target genes was effective. Next, we wanted to see if phosphatidylcholine alone was sufficient for rescue, as it ameliorates the uv1 necrosis phenotype (Crook et al. 2016). We supplemented pnc-1 animals with 0.3 mM phosphatidylcholine but found no effect on the pnc-1 egg-laying phenotype (Fig. 1b).
We have shown that overactivation of the C. elegans let-23 EGF receptor robustly rescues the pnc-1 egg-laying phenotype, but that gain-of-function mutations in the known downstream signaling mediators let-60/ Ras and itr-1 are not sufficient. Phosphatidylcholine synthesis is not required for the let-23(sa62)gf rescue of the egg-laying phenotype and phosphatidylcholine supplementation of pnc-1 had no significant effect at the sample sizes used, in contrast to the role of phosphatidylcholine in let-23(sa62)gf rescue of uv1 necrosis. We have clearly demonstrated another role for let-23 outside that of growth and development. However, the mechanism by which overactive LET-23 rescues egg-laying in pnc-1 animals is not clear. LET-23 may act via an as yet unknown pathway that restores uterine or vulval muscle function by either reducing the production of nicotinamide in those tissues or promoting some other compensatory mechanism.
Reagents
Strains:
N2 Bristol
BL5715 inIs179 (ida-1::gfp) II
HV560 inIs179 (ida-1::gfp) II; pnc-1(pk9605) IV
HV639 inIs179 (ida-1::gfp) II; pnc-1(pk9605) let-60(n1046gf) itr-1(sy290gf) unc-24(e138) IV
HV662 inIs179 (ida-1::gfp) II; pnc-1(pk9605) let-60(n1046gf) IV
HV663 inIs179 (ida-1::gfp) II; pnc-1(pk9605) itr-1(sy290gf) unc-24(e138) IV
HV776 let-23(sa62gf) inIs179(ida-1p::gfp) II; pnc-1(pk9605) IV
The strains used in this study are available from the authors upon request.
We used the following clones from the Ahringer RNAi library: pmt-1 ZK622.3 II-4G04, pcyt-1 F08C6.2 X-3N20, unc-22 ZK617.1 IV-6K06.
Acknowledgments
Some strains used to make the strains in this study were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
Funding
R01GM086786
Reviewed By
AnonymousHistory
Received: September 13, 2021Revision received: September 22, 2021
Accepted: September 23, 2021
Published: October 4, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Crook, M; Hanna-Rose, W (2021). Overactive EGF signaling suppresses a C. elegans pnc-1 egg-laying phenotype independent of known signaling mediators.. microPublication Biology. 10.17912/micropub.biology.000482.Download: RIS BibTeX