Metabolic Disorders and Complications Program, and Brain Repair and Integrative Neuroscience Program, Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada
Division of Experimental Medicine, Department of Medicine, McGill University, Montreal, Quebec, Canada
Department of Genetics, Harvard Medical School, Boston, MA, USA
Abstract
The mitochondrial unfolded protein response (mitoUPR) is an evolutionarily conserved pathway that restores homeostasis to the mitochondria after various disturbances. This pathway has roles in both resistance to exogenous stressors and longevity. The mitoUPR is mediated by the transcription factor ATFS-1/ATF-5, which modulates the expression of genes involved in protein folding, metabolism and stress resistance. MitoUPR activation in C. elegans is most commonly evaluated through transcriptional reporter strains for the mitochondrial chaperones HSP-6 and HSP-60. In order to obtain a more comprehensive view of transcriptional changes resulting from activation of the mitoUPR, we compared gene expression changes from three different mitoUPR-activating interventions: mutation of nuo-6, RNA interference (RNAi) knockdown of spg-7, and constitutive activation of ATFS-1. We specifically focused on gene expression changes that are dependent on ATFS-1. From this comparison, we identified 61 high confidence target genes that can be used to monitor mitoUPR activation. Notably, neither hsp-6 nor hsp-60 were significantly upregulated under all three mitoUPR activating conditions. We ranked the 61 genes according to the magnitude of upregulation and identify multiple genes that may serve as robust readouts of mitoUPR activation.
Description
The mitochondrial unfolded protein response (mitoUPR) is a stress response pathway that promotes cell survival and restores mitochondrial function when mitochondrial health is compromised (Haynes et al. 2013; Jovaisaite et al. 2014; Shpilka and Haynes 2018). While a mitoUPR was first reported in mammalian cells (Zhao et al. 2002), the initial work on the mitoUPR in C. elegans was performed by Yoneda et al. who found that treatment with ethidium bromide, which affects the replication and expression of mitochondrial DNA, increased the expression of the mitochondrial chaperone gene hsp-6 (Yoneda et al. 2004). Based on this observation, they generated hsp-6p::gfp and hsp-60p::gfp reporter strains to further study the mitoUPR. They found that either RNA interference (RNAi) targeting spg-7, the worm homolog of paraplegin, or RNAi targeting other genes encoding mitochondrial proteins resulted in activation of the hsp-6p::gfp and hsp-60p::gfp reporter strains (Yoneda et al. 2004).
The hsp-6p::gfp and hsp-60p::gfp reporter strains were used to identify other components of the mitoUPR including the transcriptional regulator UBL-5, the transcription factor DVE-1, the protease ClpP, the protein import channel HAF-1, and the transcription factor ATFS-1 (Benedetti et al. 2006; Haynes et al. 2007; Haynes et al. 2010; Nargund et al. 2012). Under normal conditions, the mitoUPR transcription factor, ATFS-1, is imported into the mitochondria through the HAF-1 import channel and degraded by the protease ClpP. However, when mitochondria are impaired, ATFS-1 import into the mitochondria is blocked. Instead, the nuclear localization signal (NLS) of ATFS-1 drives ATFS-1 into the nucleus. In the nucleus, ATFS-1 acts with DVE-1 and UBL-5 to upregulate genes that restore mitochondrial homeostasis, including genes involved in mitochondrial protein folding and metabolism. In addition to responding to disruptions in mitochondrial function and integrity, the mitoUPR also plays important roles in resistance to stress (Pellegrino et al. 2014; Campos et al. 2021; Soo et al. 2021) and longevity (Durieux et al. 2011; Houtkooper et al. 2013; Bennett et al. 2014; Wu et al. 2018).
Previous studies from our laboratory and others have used RNA sequencing (RNA-seq) or microarrays to examine gene expression under conditions that induce ATFS-1 activation. Nargund et al. identified 366 genes that are upregulated by spg-7 RNAi in an ATFS-1-dependent manner (Nargund et al. 2012). We previously identified 1704 genes that are upregulated in nuo-6 mutants in an ATFS-1-dependent manner (Senchuk et al. 2018; Wu et al. 2018) and 529 genes that are upregulated in both atfs-1(et15) and atfs-1(et17) constitutively active atfs-1 mutants (Rauthan et al. 2013; Wu et al. 2018).
In order to establish a list of mitoUPR target genes that are consistently upregulated across all three ATFS-1-activating conditions, we examined the overlap between these datasets. We identified a total of 61 genes that are upregulated in nuo-6 worms in an ATFS-1-dependent manner, upregulated by spg-7 RNAi in an ATFS-1-dependent manner, and upregulated in both atfs-1(et15) and atfs-1(et17) constitutively active mutants (Extended data, Column A,B). Surprisingly, neither hsp-6 nor hsp-60 were among the 61 genes on this list. In fact, hsp-60 was not identified in any of the three datasets. It is uncertain why spg-7 RNAi increases fluorescence in the hsp-60p::GFP reporter strain (Yoneda et al. 2004) but hsp-60 was not identified as one of the genes upregulated by spg-7 RNAi (Nargund et al. 2012). One possibility may be that hsp-60 expression is primarily upregulated during development in response to spg-7 RNAi and other mitochondrial insults. Then the half-life of the HSP-60::GFP protein might allow the increase in HSP-60::GFP to still be observed at adulthood even after the hsp-60 mRNA has returned to baseline.
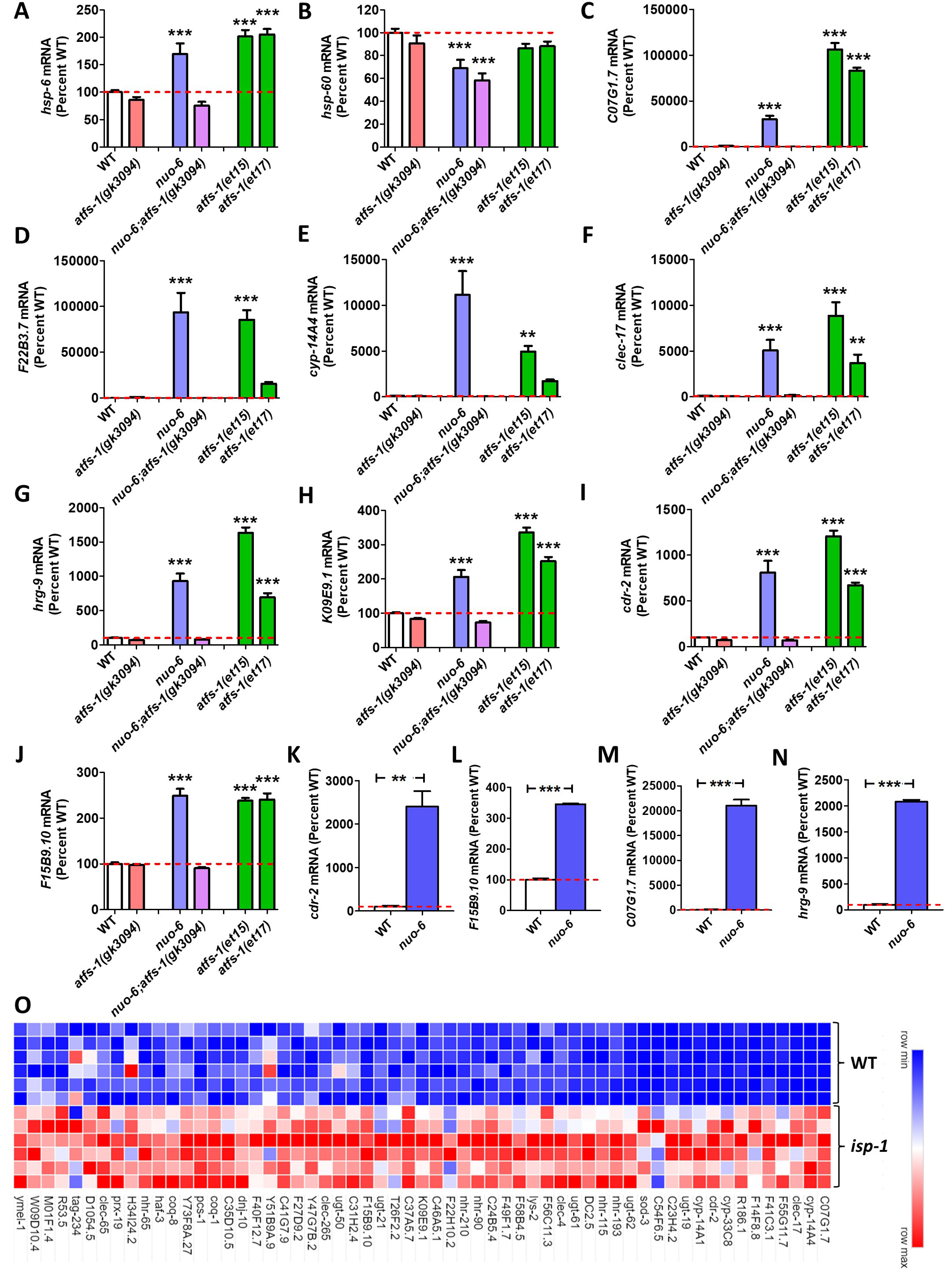
In order to rank the different mitoUPR target genes, each gene was scored by adding together their expression levels in nuo-6, atfs-1(et15) and atfs-1(et17) mutants and subtracting their expression level in nuo-6;atfs-1 mutants. By this metric, 42 of the 61 mitoUPR target genes exhibited a higher score than hsp-6 (Extended data, Column J). The top genes of C07G1.7, F22B3.7, cyp-14A4, and clec-17 had scores ranging from 17084-21882, compared to a score of 350 for hsp-6. The expression of these genes compared to hsp-6 and hsp-60 is shown in Fig 1A-F.
In order to control for variability, we divided this score by the average standard deviation from WT, atfs-1(gk3094), nuo-6, nuo-6;atfs-1(gk3094), afts-1(et15) and atfs-1(et17). By this metric, 27 of the 61 mitoUPR target genes showed a higher score than hsp-6 (Extended data, Column K). The top genes of C07G1.7, hrg-9, K09E9.1, cdr-2 and F15B9.10 had variability-corrected scores of 26-38 compared to 15 for hsp-6. The expression of these genes is shown in Fig 1G-J.
To determine if these mitoUPR target genes are being activated directly by binding of ATFS-1, we examined the data from a previous chromatin immunoprecipitation sequencing (ChIP-seq) experiment that identified genes bound by ATFS-1 after treatment with spg-7 RNAi (Nargund et al. 2015). We found that 22 of the 61 mitoUPR target genes exhibited binding of ATFS-1 after spg-7 RNAi (Extended data, Column L). This suggests that the expression of these genes is directly modulated by ATFS-1, while the expression of the other mitoUPR target genes may be regulated indirectly, or perhaps bound by ATFS-1 under different conditions than were utilized in the ChIP-seq study.
Our results suggest that the genes identified here may be better for monitoring the activation of the mitoUPR by quantitative RT-PCR or RNA-seq than hsp-6 and hsp-60. To that end, we have designed and validated primers for quantitative RT-PCR to measure the levels of two of the highest-ranked direct targets, cdr-2 and F15B9.10, and two of the highest ranked indirect targets, C07G1.7 and hrg-9. We confirmed that all four sets of primers can efficiently measure activation of the mitoUPR in nuo-6 worms compared to wild-type worms (Fig 1K-N).
Finally, to validate this list of 61 genes for monitoring the activation of the mitoUPR, we examined the long-lived mitochondrial mutant isp-1, which we have previously shown to have increased activation of a hsp-6p:::gfp reporter strain and significantly overlapping gene expression changes with constitutively active atfs-1 mutants (Wu et al. 2018). 57 of the 61 genes were found to be significantly upregulated in isp-1 mutants (Extended data, Fig 1O).
Overall, this work has identified a gene expression signature that can be used to monitor the activation of the mitoUPR in RNA-seq, microarray or qPCR studies. These genes can be used to complement the use of hsp-6p::gfp and hsp-60p::gfp reporter strains, and may provide a more robust measure of mitoUPR activation in studies measuring mRNA.
Methods
Request a detailed protocolStrains. Worms were grown on NGM plates seeded with OP50 bacteria at 20°C. We previously performed RNA sequencing on wild-type(N2), atfs-1(gk3094), nuo-6(qm200), nuo-6(qm200);atfs-1(gk3094), atfs-1(et15), atfs-1(et17) and isp-1(qm150) worms (Senchuk et al. 2018; Wu et al. 2018). This RNA sequencing data was re-analyzed to generate Figure 1, panels A-J and Figure 1, panel O. The quantitative RT-PCR results in Figure 1, panels K-N were generated for this publication using wild- type and nuo-6(qm200) worms
Quantitative RT-PCR. Worms from an overnight limited lay were allowed to grow to the prefertile young adult stage. Worms were washed three times with M9 buffer and frozen in Trizol. RNA was isolated by phenol-chloroform extraction using Trizol reagent as previously described (Machiela et al. 2016). RNA was converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (Life Technologies) according to the manufacturer’s protocol. qPCR was performed in a Viia 7 RT-PCR machine from Applied Biosystems real-time thermal cycler using PowerUp SYBR Green Master Mix kit (Applied Biosystems). RNA was collected from three biological replicates and normalized to the levels of act-3. Primer sequences:
cdr-2 (L: CGAGCCTCATTTGGAAAGAA, R: GCATCTGCCGCTGTAACTTT)
F15B9.10 (L: CCGGACAGTTTCAAGAATGC, R: CACTGAGGATCCAATGTCCA)
hrg-9 (L: TGGAATATTGAGTGGCGTTG, R: CCTCCTCTACTTGGTGCATGT)
C07G1.7 (L: GCTGAAGAAGCTTCAACCGTAG, R: TCTCGTGTCAATTCCGGTCT)
Analysis of gene expression data. Lists of differentially expressed genes were obtained from (Nargund et al. 2012;Wu et al. 2018). The raw data from these experiments is available on NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE38196, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE110984). We used BioVenn (https://www.biovenn.nl/index.php) to generate lists of overlapping genes between gene sets. Genes upregulated in nuo-6 worms in an ATFS-1-dependent manner are genes that are upregulated in nuo-6 mutants but not nuo-6;atfs-1 mutants. To rank mitoUPR target genes, expression of each gene was normalized to wild-type. The score for each gene was determined by summing the normalized expression in nuo-6, atfs-1(et15) and atfs-1(et17) mutants and subtracting three times the expression in nuo-6;atfs-1 mutants. To generate a variability-corrected score, this score was divided by the average standard deviation for each of the strains. Binding of ATFS-1 was determined from a previous chromatin immunoprecipitation (ChIP) study (Nargund et al. 2015). Data from this study is available on NCBI GEO: (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE63803).
Reagents
| Strain | Genotype | Source |
| N2 | wild-type | Wild isolate from Bristol |
| MQ1333 | nuo-6(qm200) | Yang, Hekimi. 2010. |
| VC3201 | atfs-1(gk3094) | C. elegans Reverse Genetics Core Facility at the University of British Columbia |
| QC115 | atfs-1(et15) | Rauthan et al. 2013. |
| QC117 | atfs-1(et17) | Rauthan et al. 2013. |
| MQ887 | isp-1(qm150) | Feng et al. 2001. |
| JVR477 | nuo-6(qm200); atfs-1(gk3094) | Wu et al. 2018. |
Acknowledgments
We would like to thank Dr. Paige Rudich for reviewing this manuscript and providing suggestions for improvement. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P30 OD010440). We would also like to acknowledge the C. elegans knockout consortium and the National Bioresource Project of Japan for providing strains used in this research.
Extended Data
Van Raamsdonk, J. (2021). List of ATFS-1 targets (Version 1.0) [Data set]. CaltechDATA 10.22002/D1.2130
References
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR; http://www.cihr-irsc.gc.ca/; JVR) and the Natural Sciences and Engineering Research Council of Canada (NSERC; https://www.nserc-crsng.gc.ca/index_eng.asp; JVR) and the National Institute of General Medical Sciences (NIGMS; https://www.nigms.nih.gov/; JVR) by grant number R01 GM121756. JVR received a Senior Research Scholar career award from the Fonds de Recherche du Quebec Santé (FRQS) and Parkinson Quebec. SKS received a scholarship from FRQS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Reviewed By
AnonymousHistory
Received: September 15, 2021Revision received: September 23, 2021
Accepted: September 27, 2021
Published: October 19, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Soo, SK; Van Raamsdonk, JM (2021). High confidence ATFS-1 target genes for quantifying activation of the mitochondrial unfolded protein response. microPublication Biology. 10.17912/micropub.biology.000484.Download: RIS BibTeX