UBC/LSI Bioinformatics Facility, University of British Columbia, Vancouver, British Columbia, Canada
Abstract
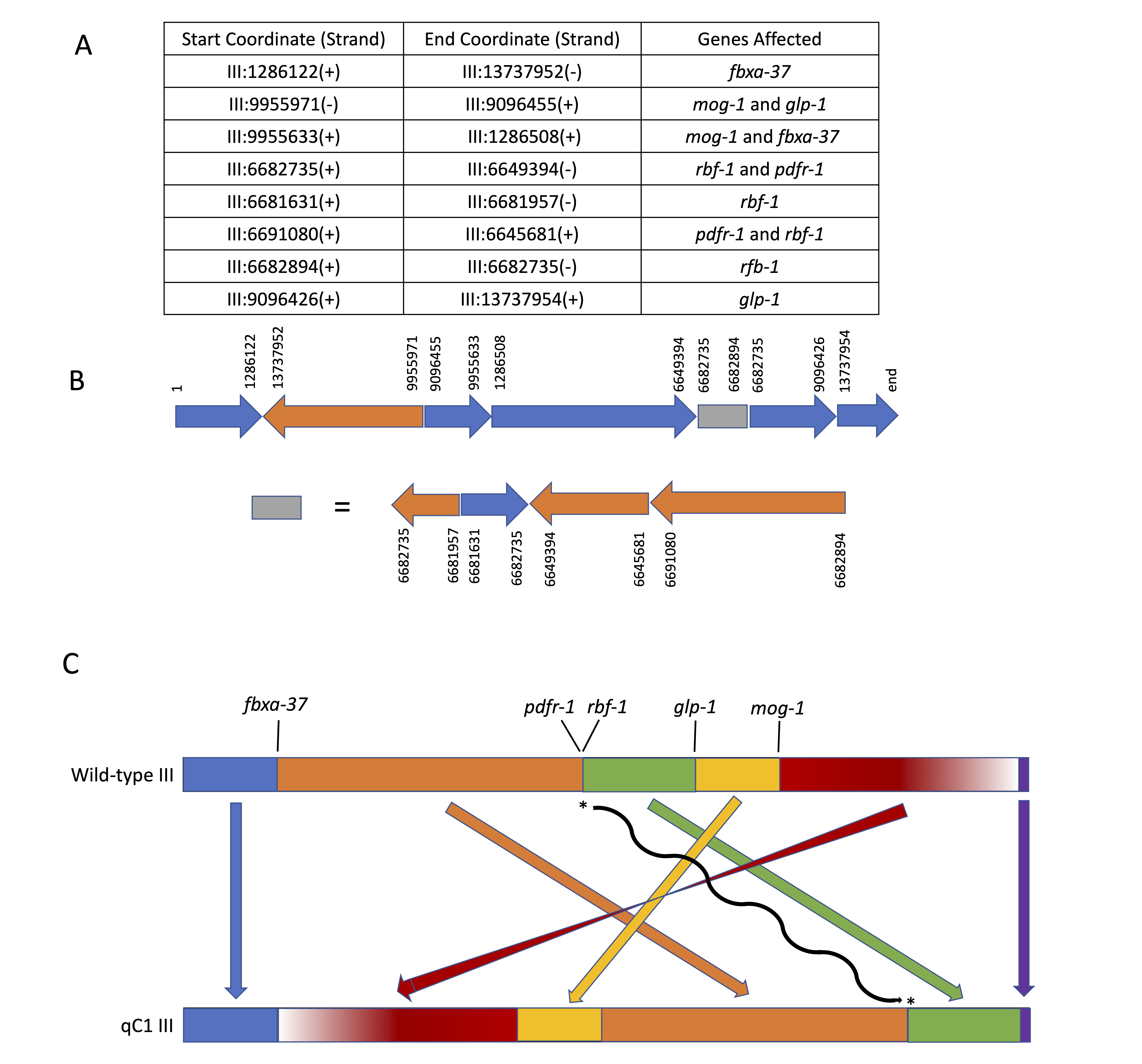
We used whole-genome sequencing (WGS) data from a number of balanced lethal strains in Caenorhabditis elegans to show that the crossover suppressor qC1 is an inversion. The rearrangement is complex, with a large primary inversion that contains several other smaller inverted regions. The graphical representation below depicts these various qC1 rearrangements for ease of conceptualization. It is the simplest chromosomal structure compatible with the data currently available, but even then it is worth noting that the complexity of the qC1 chromosome can make the graphical reconstruction difficult to understand, and it may seem a bit like relativity theory or artwork from M.C. Escher (https://moa.byu.edu/m-c-eschers-relativity/).
Description
Classical dominant intrachromosomal crossover suppressors in C. elegans (e.g. mnC1, sC1, qC1, mC6 [now mIn1]) have long been thought likely to be inversions. Prior to economical genome sequencing, proof of inversion depended on the isolation of crossover suppressor variants differentially marked with morphological mutations, construction of homozygous suppressor genotypes with different such markers in trans, and recombination analysis within and outside the putative inverted region (hIn1: Zetka and Rose, 1992; mIn1: Edgley and Riddle, 2001).
Here we report genome sequence data confirming that the crossover suppressor qC1 (Austin and Kimble, 1989) is a complex inversion (Fig. 1). The data used in this analysis were generated in the course of genome sequencing to identify causative lesions in lethal mutations balanced by qC1 (Li-Leger et al., 2021).
The primary inverted region in qC1 runs from LG III coordinates 1,286,123 through 13,737,951; these physical breakpoints correspond to the genetic limits of balancing behavior observed in recombination analysis of marker/qC1trans heterozygotes (dpy-1 to tra-1, summarized in Edgley et al., 1995). In addition to the primary inversion, the qC1chromosome is characterized by several regions exhibiting further rearrangement, possibly the result of the radiation dose used to generate the suppressor (7200 R gamma). The original balancer was known to carry lesions in glp-1 and mog-1 that were not present in the strain mutagenized to generate qC1 and not characterized molecularly; these lesions were both identified as additional inversion breakpoints inside the primary qC1 inversion. In particular, as can be seen in Fig. 1, glp-1(q339) consists of two breakpoints within the gene at chromosome coordinates 9,096,426 and 9,096,455, which in addition to breaking the gene deletes 28 base pairs from one of its coding exons. A similar event is seen in mog-1, with breakpoints at coordinates 9,955,633 and 9,955,971.
The schematic representation of qC1 chromosome III shown in Fig. 1B and1C is the simplest structure compatible with the breakpoints and breakpoint connections identified in the current study. However, we cannot exclude the possibility that additional breakpoints have been undetected, which would result in more complex chromosomal rearrangements.
Methods
Request a detailed protocolSequence Generation
Large asynchronous populations of balanced heterozygous strains were harvested by washing freshly-starved 100 mm standard agar/OP50 culture plates with M9 buffer. Worms were pelleted in 15 ml centrifuge tubes, the supernatant was removed by aspiration, and the packed worms were used to make purified DNAs using standard extraction protocols. These DNAs were subjected to sequencing on the Illumina platform and the data were subsequently analyzed in-house. The strains with qC1 balancer analyzed in the current study were sequenced at an average depth of coverage ranging from 43x to 48x (mean and median of 46x).
Sequence Analysis
Read alignments created for the study by Li-Leger et al.. (2021) were first visually inspected within genes known to be mutated in qC1 (i.e. glp-1 and mog-1) using the IGV genome viewer (Thorvaldsdóttir et al.., 2013) in order to locate obvious breakpoints in chromosome III. LUMPY (Layer et al., 2014) was then used to search for potentially missed breakpoints within the whole genome. Candidate breakpoints were kept for further investigation only when they were present in strains containing the qC1 chromosome and absent in strains without that balancer. Each breakpoint was then further analyzed by aligning split reads at the breakpoint using the blast tool available on the WormBase website (https://wormbase.org/tools/blast_blat), which allowed connecting two breakpoints to one another as reported in Fig. 1A. A mutated chromosome III for qC1 was then deduced using those breakpoint connections and a parsimonious approach while taking into account the copy-number evidence provided by the read coverage in the regions of interest. All analysis was done using the reference genome in WormBase (https://wormbase.org, WS282).
Reagents
DNA sequence analysis was based on the wild type N2 Bristol derived strain PD1074, the Million Mutation Project strain VC30189 and several qC1 and nT1 strains that were derived from a parental strain following mutagenesis with EMS (ethyl methane-sulfonate). Each of the strains resulting from mutagenesis harboured a lethal mutation in cis with unc-32 balanced by qC1 or in cis with unc-24 balanced by nT1. Further details for these strains can be obtained in Li-Leger et al.. (2021).
| Strain | Genotype | Source |
| PD1074 | N2 derivative | CGC |
| GE2220 | unc-32(e189) top-3(t1516)/qC1[dpy-19(e1259) glp-1(q339)] III; him-3(e1147) IV | Li-Leger et al., 2021 |
| GE2348 | unc-32(e189) zyg-8(t1518)/qC1[dpy-19(e1259) glp-1(q339)] III; him-3(e1147) IV | Li-Leger et al., 2021 |
| GE2352 | unc-32(e189) cyk-3(t1535)/qC1[dpy-19(e1259) glp-1(q339)] III; him-3(e1147) IV | Li-Leger et al., 2021 |
| GE2357 | unc-32(e189) cls-2(t1527)/qC1[dpy-19(e1259) glp-1(q339)] III; him-3(e1147) IV | Li-Leger et al., 2021 |
| GE2367 | unc-32(e189) klp-19(t1563)/qC1[dpy-19(e1259) glp-1(q339)] III; him-3(e1147) IV | Li-Leger et al., 2021 |
| GE1958 | him-9(e1487) II; unc-24(e138) atg-7(t1726)/nT1[let(m435)] IV; dpy-11(e224)/nT1[let(m435)] V | CGC |
| GE2391 | him-9(e1487) II; unc-24(e138) dif-1(t1932)/nT1[let(m435)] IV; dpy-11(e224)/nT1[let(m435)] V | CGC |
| GE2881 | him-9(e1487) II; unc-24(e138) F56D5.2(t1744)/nT1[let(m435)] IV; dpy-11(e224)/nT1[let(m435)] V | Li-Leger et al., 2021 |
| GE2890 | him-9(e1487) II; unc-24(e138) C34D4.4(t1821)/nT1[let(m435)] IV; dpy-11(e224)/nT1[let(m435)] V | Li-Leger et al., 2021 |
| VC30189 | Million Mutation Project strain. This strain was isolated after ENU (n-ethyl-N-nitrosourea) mutagenesis of VC2010, propagated clonally through 10 generations to drive mutations to homozygosity, and subjected to whole-genome sequencing. It is homozygous for a large number of mutations determined from sequence data. | CGC |
Acknowledgments
The authors thank Jeremy Nance for asking if we had investigated the mutations in qC1 using the sequencing reads from Li-Leger et al., which prompted the current study.
References
Funding
This work was supported by Canadian Institute of Health Research grant PJT-148549 (awarded to DGM). This work was also supported by an R24 National Institute of Health grant 5R240D023041 (awarded to Ann Rougvie, Paul Sternberg, Geraldine Seydoux and DGM).
Reviewed By
AnonymousHistory
Received: October 8, 2021Revision received: October 21, 2021
Accepted: October 26, 2021
Published: November 3, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Edgley, ML; Flibotte, S; Moerman, DG (2021). Sequenced Breakpoints of Crossover Suppressor/Inversion qC1. microPublication Biology. 10.17912/micropub.biology.000494.Download: RIS BibTeX