Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI 48824, USA
Department of Microbiology & Molecular Genetics, Michigan State University, East Lansing, MI 48824, USA
Abstract
Using a previously established transgenic approach to inactivate phytochrome chromophore synthesis in specific organs or tissues, we used a guard cell-specific promoter to induce phytochrome deficiencies in guard cells of Arabidopsis thaliana. Analyses of multiple homozygous lines depleted of phytochromes in stomatal guard cells indicated elongated hypocotyls specifically in red and far-red growth conditions. Furthermore, rosette leaves of adult plants with guard cell-specific phytochrome deficiencies showed enhanced serration compared to the wild-type Col-0 parent. Thus, we demonstrate that guard cell-localized phytochromes impact the inhibition of hypocotyl elongation, as well as leaf margin morphology of adult rosette leaves in A. thaliana.
Description
Light impacts growth and development throughout the plant life cycle; however, photomorphogenesis occurs differently in individual tissues and organs (Montgomery, 2016). Light actively inhibits elongation in the hypocotyl, but promotes the growth and development of cotyledons, leaves, and roots. Distinct responses in specific tissues are maintained to some extent through the differential accumulation of photoreceptors such as phytochromes in distinct tissues and at different times during development (Adam et al., 1994; Somers and Quail, 1995a, 1995b; Goosey et al., 1997; Nagatani, 1997; Tóth et al., 2001; Sharrock and Clack, 2002; Baba-Kasai et al., 2014; van Gelderen et al., 2018); as well as in large part due to distinct signal transduction pathways downstream of photoreceptors in distinct tissues and organs (Bou-Torrent et al., 2008; Montgomery, 2008; Endo et al., 2016; Montgomery, 2016).
Stomata are specialized epidermal cells that occur in the epidermis for all above-ground tissues and serve to facilitate gas exchange and water uptake central to photosynthesis. Stomata respond to environmental signals, including light (Roth-Bejerano and Itai, 1981; Kinoshita et al., 2001; Talbott et al., 2003; Boccalandro et al., 2009; Casson et al., 2009; Matthews et al., 2020; Zhu et al., 2020). Specific phytochromes have been shown to impact stomatal development and function, with phyB promoting the stomatal index, or the percentage of stomata cells out of the total number of epidermal cells, and regulation of stomatal opening in red light (Boccalandro et al., 2009; Casson et al., 2009; Kang et al., 2009; Wang et al., 2010; Casson and Hetherington, 2014). The photoreceptor phyA stimulates production of stomata in far-red light (Kang et al., 2009). Notably, phytochromes are localized in guard cells themselves (Somers and Quail, 1995b; Kang et al., 2009), and thus stomatal responses can potentially be controlled by photoreceptors locally or through intercellular communication.
Tissue-specific expression of PHY genes in phy mutant backgrounds has been used to explore spatial-specific roles of phytochromes. In regards to the function of phyB in regulating stomata, recent analyses used tissue-specific PHYB (At2g18790) expression to explore phytochrome-dependent roles in guard cells (Casson and Hetherington, 2014). Expression of PHYB in guard cells demonstrated that phyB accumulation in these cells was sufficient to rescue a defect in photoregulation of the stomatal index in a phyB mutant (Casson and Hetherington, 2014). These plant lines with guard cell-specific PHYB expression also exhibited larger leaves, potentially indicating more extensive growth-related impacts of guard cell-specific phytochrome function. Prior investigations also correlated stomatal regulation with control of additional whole plant growth phenotypes, including correlating the regulation of stomatal opening with vegetative development in terms of the hypocotyl length of seedlings (Xinhong et al., 2011), and the rosette and flowering time at the adult stage (Kinoshita et al., 2011; Ando et al., 2013).
To investigate the function of phytochromes in guard cells, we used a previously verified transgenic approach for expressing a phytochrome chromophore-inactivating enzyme, biliverdin reductase (BVR; UniProt: P46844) to regulate accumulation of photoactive phytochromes in planta (Lagarias et al., 1997; Montgomery et al., 1999; Montgomery et al., 2001; Warnasooriya and Montgomery, 2009; Costigan et al., 2011). We used the promoter pGC1 (At1g22690), which drives high-level, guard-cell specific gene expression and has low expression activity in mesophyll cells (Yang et al., 2008), to generate plant lines with guard cell-localized, plastid-targeted BVR (pBVR) accumulation to induce guard cell-specific phytochrome depletion. We used these lines to examine the roles of guard cell-localized phytochromes in distinct aspects of photomorphogenesis.
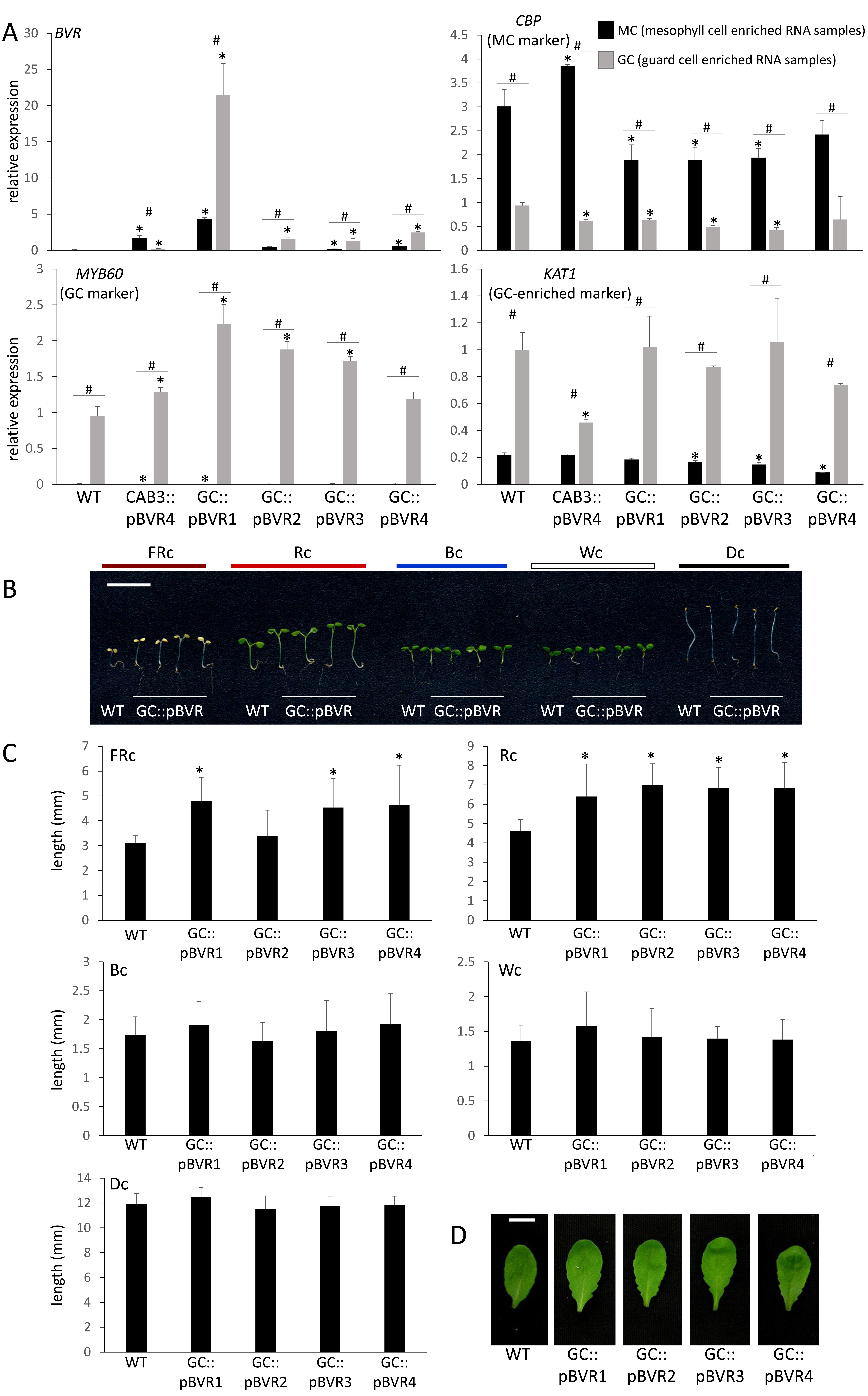
We successfully isolated four lines with guard-cell enriched expression of BVR, i.e., GC::pBVR1 to GC::pBVR4 as verified by qRT-PCR analyses with RNA extracted from mesophyll cell (MC) and guard cell enriched epidermal peel (GCEEP) fractions and compared to MC and GCEEP fractions from the CAB3::pBVR representative line with MC-localized BVR expression (Fig 1A; Warnasooriya and Montgomery, 2009). We observed significant enrichment of BVR expression in GCEEP compared to MC in several GC::pBVR lines, confirming guard-cell enriched BVR expression driven by the GC promoter. To confirm the reliability of our MC and GCEEP fractions, we tested RNA fractions for expression of an MC-enriched marker gene, i.e., CALMODULIN-BINDING PROTEIN (CBP; At4g33050) (Cho et al., 2012), and guard-cell marker genes, i.e., POTASSIUM (K+) CHANNEL IN ARABIDOPSIS THALIANA 1 (KAT1; At5g46240) (Pilot et al., 2001) and MYB DOMAIN PROTEIN 60 (MYB60; At1g08810) (Cominelli et al., 2005). As expected, we detected preferential expression of CBP in the MC RNA enrichment and of KAT1 and MYB60 in the GCEEP RNA enrichment (Fig 1A). Additionally, we assessed protein extracts from GCEEP for BVR enzymatic activity using a previously described specific activity assay (Lagarias et al., 1997). Whereas no BVR activity was detected for GCEEP protein extracts from WT and a CAB3::pBVR line, we detected BVR activity in the range of 0.2 – 2.8 (I.U. mg−1) × 103 for GCEEP extracts from GC::pBVR lines.
We assessed light-dependent growth of GC::pBVR seedlings compared to Col-0 WT seedlings under constant blue (Bc), red (Rc), far-red (FRc) and white (Wc) light. We observed significantly elongated GC::pBVR seedlings under both FRc and Rc illumination (Fig 1B and 1C). GC::pBVR seedlings were not significantly different relative to WT seedlings in the dark or under either Bc or Wc light (Fig 1B and 1C), which is distinct from phenotypes observed due to depletion of phytochromes in mesophyll cells as CAB3::pBVR lines were previously reported to have elongated hypocotyls in Wc, FRc, Rc, and Bc (Warnasooriya and Montgomery, 2009; Warnasooriya et al., 2011). The disruption in light-dependent inhibition of hypocotyl elongation only under R and FR light for GC::pBVR lines is notable as these are wavelengths specifically associated with phytochrome-regulated functions. Although not previously associated specifically with light-dependent regulation, prior analyses of plants with altered ABA-regulated stomatal function showed a correlation between higher rates of stomatal closure and elongated hypocotyls (Xinhong et al., 2011).
For adult plants, GC::pBVR plants exhibited altered leaf shape (i.e., enhanced serration) under long-day conditions of W light at 100 µmol m-2 s-1 (Fig 1D). In Arabidopsis, leaf heteroblasty can be indicated by leaf margin serration. As the major disruption at the adult leaf stage for GC::pBVR lines was an alteration in leaf serration or leaf margin morphology defects, these plants may exhibit alterations in heteroblasty correlated with guard-cell phytochrome deficiency. Relatedly, prior analyses with tissue-specific expression of phyB also indicated a correlation between a role for guard cell-localized phyB and leaf phenotypes. The leaves of phyB mutants are significantly different from WT with less leaf area and elongated petioles (Casson and Hetherington, 2014). When a phyB mutant was specifically complemented with a guard cell-localized PHYB construct, i.e., SPCH::PHYB, the leaf phenotype was largely complemented, in addition to the stomatal index phenotype (Casson and Hetherington, 2014). Together, these associations of the regulation of leaf phenotypes with stomatal phytochrome pools indicate key roles of GC-localized phytochromes in regulating whole leaf phenotypes.
Methods
Request a detailed protocolPlasmid construction
We constructed plant transformation vector pORE_O3_GC/TPBVR using a TP-BVR fragment for plastid-targeted BVR protein accumulation from the previously described CAB3::pBVR construct pBIB/CAB3-TPBVR (Warnasooriya and Montgomery, 2009) and guard cell-specific GC promoter (Yang et al., 2008). Specifically, the guard-cell specific, plastid-targeted BVR construct (GC::pBVR) was made by isolating the transit peptide-BVR (pBVR) fragment from pBIB/CAB3-TPBVR using XbaI and SacI and subcloning into XbaI and SacI digested pORE_O3_GC plasmid, which contains the GC1 promoter [pGC1 (At1g22690)], by ligation to create the pORE_O3_GC/TPBVR plasmid.
Plant transformation and growth conditions
Arabidopsis thaliana (Columbia or Col-0 ecotype) plants were used as wild-type (WT) and for transformation of all constructs. We used both pBIB/CAB3-TPBVR and pORE_O3_GC/TPBVR constructs in Agrobacterium tumefaciens strain GV3101. We isolated multiple single insertion homozygous lines that were screened for BVR expression, including enrichment in the mesophyll or guard cells for the CAB3::pBVR4 (also denoted as CAB3::pBVR as the sole line used in these analyses) and GC::pBVR lines, respectively.
For hypocotyl growth analyses, surface sterilized seeds were plated and grown on MS medium containing 1% (w/v) sucrose and 0.7% (w/v) Phytoblend agar (Caisson Labs, UT) at 22°C for 7 d under the indicated light conditions. Light sources used included blue (B; λmax ∼470 nm), far-red (FR; λmax ∼735 nm), red (R; λmax ∼670 nm), and white (W) as previously described (Warnasooriya and Montgomery, 2009). Light intensities were measured using a LI-COR LI-250A Light Meter with inline LI-COR quantum sensor for R, B and W light, and using a StellarNet EPP2000 spectroradiometer (Apogee Instruments) for FR light.
Phenotyping
We determined light-dependent hypocotyl elongation of seedlings under specified growth conditions by scanning images of seedlings and quantifying hypocotyl length using ImageJ software as previously described (Warnasooriya and Montgomery, 2009). For phenotypic observation of rosette leaf morphology, plants were grown in a long-day growth chamber with 16 h/8 h day/night light cycle at a white light intensity of ~100 µmol m-2 s-1 and 22°C/20°C day/night temperature cycles.
Gene expression analysis
RNA samples were extracted using the RNeasy® Plant Minikit (Qiagen, CA) as previously described (Oh and Montgomery, 2013) from whole plants, mesophyll cells from ~15 g of 5 to 6 week-old plant leaves (Yoo et al., 2007) or guard cell-enriched epidermal peels from adult plants grown in a short-day growth chamber with 8 h/16 h day/night light cycles at a white light intensity of 100 µmol m-2 s-1 and 22°C/20°C day/night temperature cycle, according to the protocol of (Zhu et al., 2016). Quantitative RT-PCR (qRT-PCR) was performed essentially as described (Oh and Montgomery, 2013). Expression of BVR and tissue-specific marker genes was assessed, including mesophyll cell-enriched marker CBP (At4g33050) and guard cell marker genes KAT1 (At5g46240) and MYB60 (At1g08810). The control genes used for qRT-PCR analyses were PP2A (At1g13320) and ACT2 (At3g18780). Primers for each gene are listed in Table 1.
Reagents
Table 1. Primers for quantitative reverse transcriptase PCR (qRT-PCR)
| Forward primer (5ˈ−>3ˈ) | Reverse primer (5ˈ−>3ˈ) | |
| BVR | ACAAGGGTCTGCTGTCATGG | GGGACCCAGAAGTGAACTGG |
| CBP
(MC marker) |
TGTGTTTGATACCCACACAAGGG | ACCCAACTTCAAGACCCATGACC |
| MYB60
(GC marker) |
ACACTGGGTTATTGAGATGCAGCA | TGGACGCCCATTTGTTACCCAA |
| KAT1
(GC marker) |
GACGCTGAGTATTTCCCACCAA | GAAGTCCACTGCTCCTGACA |
| PP2A
(internal control) |
TAACGTGGCCAAAATGATGC | GTTCTCCACAACCGCTTGGT |
| ACT2
(internal control) |
AGCACCCTGTTCTTCTTACCG | CCAGAATCCAGCACAATACCGG |
Acknowledgments
We wish to thank Kelsey Mulvihill, who worked on this project with the financial support of the Professorial Assistantship (PA) program in MSU’s Honors College, for assistance with cloning and construction preparation, selection of homozygous GC::pBVR lines, and plant phenotyping. We also thank Dr. Sarah Assmann and members of her laboratory for providing the GC promoter and protocols and assistance with isolating guard cell epidermal peels.
References
Funding
This work was supported by the National Science Foundation (grant no. MCB-1515002 to B.L.M.) and the United States Department of Energy (Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, grant no. DE-FG02-91ER20021 to B.L.M.)
Reviewed By
Dior KelleyHistory
Received: November 29, 2021Revision received: January 17, 2022
Accepted: January 24, 2022
Published: January 31, 2022
Copyright
© 2022 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Oh, S; Kong, Q; Montgomery, BL (2022). Guard-cell phytochromes impact seedling photomorphogenesis and rosette leaf morphology. microPublication Biology. 10.17912/micropub.biology.000521.Download: RIS BibTeX