Tsinghua Institute of Multidisciplinary Biomedical Research, Tsinghua University, Beijing, China
Abstract
In Schizosaccharomyces pombe, the can1-1 mutation confers resistance to the toxic arginine analog canavanine. This mutation has been assumed to disrupt a gene encoding an arginine transporter. In PomBase, the gene SPBC18H10.16 is currently designated can1. Here, we sequenced the genomes of three can1-1 strains. No mutations were found in SPBC18H10.16. Instead, these strains harbor an R175C mutation in the gene any1 (SPBC18H10.20c). any1 encodes an α-arrestin that acts as a ubiquitin ligase adaptor to downregulate plasma membrane amino acid transporters. Our findings indicate that can1-1 is not a loss-of-function mutation in an amino acid transporter gene, but a possible gain-of-function mutation in a gene encoding a negative regulator of amino acid transporters.
Description
Canavanine is a toxic arginine analog first isolated from jack bean (Canavalia ensiformis) (Kitagawa and Yamada 1932). Because the toxicity of canavanine depends on its cellular uptake mediated by plasma membrane arginine transporters, the isolation of canavanine-resistant mutants in model microorganisms yielded pioneering discoveries on arginine transporter genes (Schwartz et al. 1959; Grenson et al. 1966). In the budding yeast Saccharomyces cerevisiae, all canavanine-resistant mutants isolated are loss-of-function mutants of the arginine transporter gene CAN1 (Whelan et al. 1979; Hoffmann 1985). Canavanine-based counter selection of CAN1 has been widely used as an assay for studying mutagenesis and genome instability in S. cerevisiae (Drake 1991; Tishkoff et al. 1997; Hackett et al. 2001; Lang and Murray 2008; Kim et al. 2011; Jiang et al. 2021).
In the fission yeast Schizosaccharomyces pombe, canavanine-resistant mutants were first isolated at the Institute of General Microbiology, University of Bern, Switzerland in the 1970s and the phenotype-causing mutations in these mutants were mapped to two loci, designated can1 and can2 (Kohli et al. 1977). A mutation at the can1 locus, can1-1, was shown to cause both canavanine resistance and reduced arginine uptake (Fantes and Creanor 1984). The canavanine resistance phenotype of the can1-1 mutant can be reversed by the expression of the S. cerevisiae CAN1 gene (Ekwall and Ruusala 1991). These observations have led to the assumption that can1-1 is a loss-of-function mutation in an S. pombe arginine transporter gene (Fantes and Creanor 1984; Ekwall and Ruusala 1991). In PomBase (Lock et al. 2019), the gene name can1 has been assigned to the gene SPBC18H10.16. This gene has not been subjected to in-depth experimental characterization. The nature of the can1-1 mutation has not been reported.
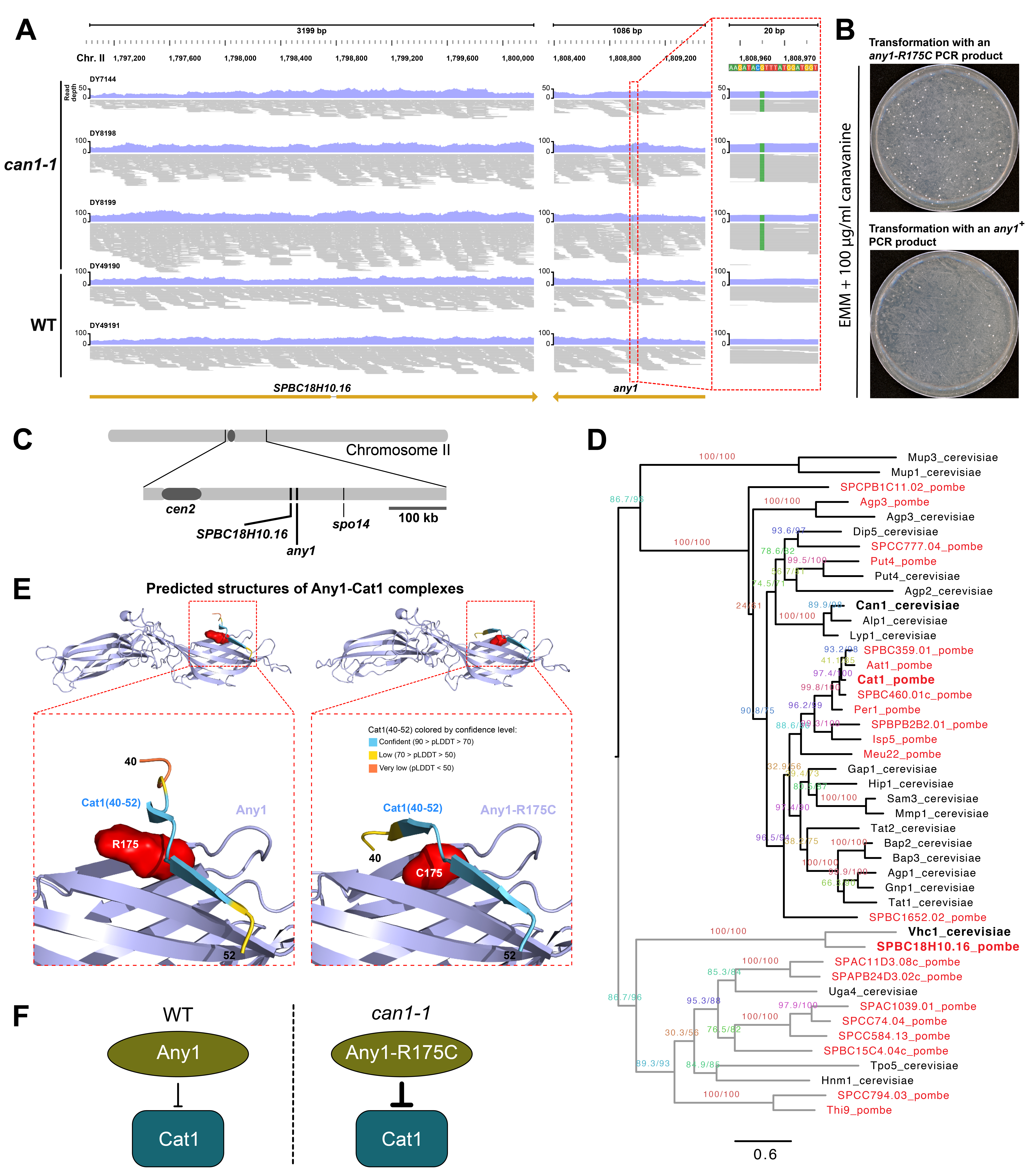
Here, we applied whole-genome sequencing to identify mutations in three S. pombe can1-1 mutant strains. To our surprise, no mutations were found in the gene SPBC18H10.16 (Figure 1A). Instead, the only mutation found in all three can1-1 strains but not in two wild-type strains is a G-to-A mutation at position 1808965 of chromosome II in the PomBase reference genome, which results in an arginine-to-cysteine (R175C) missense mutation in the gene any1 (SPBC18H10.20c) (Figure 1A). Transformation experiments showed that the any1-R175C mutation can indeed cause canavanine resistance (Figure 1B). This unexpected finding led us to investigate the possible reason behind the assignment of the name can1 to SPBC18H10.16. We were not able to find any published evidence directly demonstrating that the can1 locus corresponds to SPBC18H10.16. In the last comprehensive genetic map of S. pombe (Munz et al. 1989), the can1 locus was mapped to the interval between the centromere of chromosome II (cen2) and the spo14 gene on the right arm of chromosome II. Both SPBC18H10.16 and any1 are within this interval and are only 8.2 kb away from each other (Figure 1C). According to PomBase, the cen2–spo14 interval contains 106 protein-coding genes, among which SPBC18H10.16 is the only one encoding a protein with similarity to amino acid transporters. Thus, we surmise that the PomBase gene name assignment for SPBC18H10.16 was probably based on the genetic mapping position of the can1 locus and the assumption that this locus encodes an amino acid transporter.
The similarity between the SPBC18H10.16 protein and experimentally characterized amino acid transporters is limited to an approximately 450-amino-acid domain named “amino acid/polyamine transporter I” (CATH-Gene3D database entry G3DSA:1.20.1740.10) (Sillitoe et al. 2021). To clarify whether SPBC18H10.16 is an amino acid transporter, we performed a phylogenetic analysis (Figure 1D). This analysis showed that S. cerevisiae and S. pombe proteins containing the G3DSA:1.20.1740.10 domain fall into two families. In the family of which S. cerevisiae Can1 is a member (black branches in the phylogenetic tree in Figure 1D), all experimentally characterized family members are amino acid transporters. In contrast, in the other family of which SPBC18H10.16 is a member (grey branches in the phylogenetic tree in Figure 1D), all experimentally characterized members are transporters of non-amino-acid compounds (Vhc1, Uga4, Tpo5, Hnm1 of S. cerevisiae are transporters of metal and chloride ions, gamma-aminobutyrate, polyamine, and choline, respectively, and Thi9 of S. pombe is a transporter of thiamine). In particular, this analysis showed that SPBC18H10.16 is an ortholog of S. cerevisiae Vhc1, a cation–chloride cotransporter localized at the vacuole membrane (Petrezselyova et al. 2013). Consistent with this orthology, SPBC18H10.16 has been shown to localize at the vacuole membrane in S. pombe (Liu et al. 2015). Thus, SPBC18H10.16 encodes not a plasma membrane amino acid transporter but a vacuole membrane cation–chloride cotransporter. We propose that SPBC18H10.16 should be renamed vhc1.
The any1 gene in S. pombe encodes an α-arrestin. α-arrestins are ubiquitin ligase adaptors that target plasma membrane transporters for ubiquitination by the NEDD4/Rsp5 family ubiquitin ligases, and thereby reduce the levels of transporters at the plasma membrane through altering their trafficking (Alvarez 2008; Lin et al. 2008; O’Donnell and Schmidt 2019; Kahlhofer et al. 2021). In S. pombe, Any1 negatively regulates two plasma membrane amino acid transporters Aat1 and Cat1 (Nakase et al. 2013; Nakashima et al. 2014). Cat1 is the major cationic amino acid transporter in S. pombe, and loss of Cat1 results in canavanine resistance and a severe reduction of arginine uptake (Aspuria and Tamanoi 2008). Interestingly, despite sharing similar roles in arginine transport and canavanine toxicity, S. pombe Cat1 and S. cerevisiae Can1 are not orthologs (Figure 1D). Instead, they belong to two distinct subfamilies that have each undergone lineage-specific expansion (Figure 1D). Consistent with the function of Any1 in downregulating Cat1, deletion of any1 renders S. pombe cells more sensitive to canavanine, whereas overexpression of any1 confers canavanine resistance (Nakase et al. 2013; Nakashima et al. 2014). Because the any1-R175C (can1-1) mutation shares the same phenotypic consequence as the overexpression of any1, we propose that the any1-R175C mutation is likely a gain-of-function mutation.
To explore the possible reason behind the gain-of-function effect of the any1-R175C mutation, we examined how Any1 may interact with Cat1 by applying the protein complex structure prediction tool AlphaFold-Multimer (Evans et al. 2021). The prediction result shows that a short stretch of 10 amino acids (residues 43-52) in the N-terminal cytoplasmic tail of Cat1 interacts with the arrestin-N domain of Any1 (Figure 1E). This stretch is unstructured (pLDDT < 50) in the AlphaFold-predicted structure of Cat1 alone (Jumper et al. 2021; Varadi et al. 2022). Intriguingly, the R175 residue of Any1 is located at the predicted interaction interface. This prompted us to predict the structure of the Any1-R175C mutant protein in complex with Cat1. Remarkably, in the mutant complex, three more residues of Cat1 (residues 40-42) become structured and make contacts with Any1 (Figure 1E). The interface area increases from 578.6 Å2 in the wild-type complex to 739.2 Å2 in the mutant complex, and the free energy of dissociation (energy required to dissociate the two proteins) increases from 2.0 kcal/mol for the wild-type complex to 3.7 kcal/mol for the mutant complex, suggesting that the R175C mutation enables Any1 to interact more strongly with Cat1. These structure prediction results support a model that the any1-R175C (can1-1) mutation is likely a gain-of-function mutation that results in stronger downregulation of Cat1 (Figure 1F).
The proposed gain-of-function nature of the any1-R175C (can1-1) mutation is consistent with the fact that can1-1 is a semi-dominant mutation in that can1-1/can1+ heterozygous diploid cells are substantially more resistant to canavanine than can1+/can1+ diploid cells and slightly less resistant than can1-1/can1-1 diploid cells (Fantes and Creanor 1984). This is different from the situation in S. cerevisiae, where all canavanine-resistant mutations (loss-of-function can1 mutations) are recessive (Whelan et al. 1979). The reversal of the canavanine resistance phenotype of the can1-1 mutant by the expression of the S. cerevisiae CAN1 gene (Ekwall and Ruusala 1991) can be explained by an inability of Any1-R175C to downregulate S. cerevisiae Can1, as the Any1-interacting region of S. pombe Cat1 predicted by AlphaFold-Multimer is not conserved in S. cerevisiae Can1.
The frequency of canavanine-resistant mutations has been used as a readout of mutation rate in S. pombe (Kaur et al. 1999; Fraser et al. 2003; Tanaka et al. 2004; Sabatinos et al. 2013). The clarification of the identity of the gene affected by the can1-1 mutation will benefit the design and interpretation of canavanine resistance-based analyses of mutation rates and mutation spectra. Loss-of-function mutations in the cat1 gene are likely to be much more common than gain-of-function mutations in the any1 gene among spontaneous and mutagen-induced canavanine-resistant S. pombe mutations.
Methods
Request a detailed protocolWhole genome sequencing and sequencing data analysis: Tagmentation-based sequencing library preparation was performed as described previously (Tao et al. 2019). Paired-end 2 × 150 bp reads were generated on the Illumina NovaSeq 6000 platform. FASTQ files were pre-processed by cutadapt (version 3.3) with the options –gc-content=36 -q 30,30 –trim-n -m 50 –pair-filter=any (Martin 2011). Potential cross-contaminating reads that harbor 27-mers with frequencies lower than 20% of the peak frequency of 27-mers were identified and filtered out using KAT (Mapleson et al. 2017). The pre-processed and filtered reads were mapped to the S. pombe reference genome (https://www.pombase.org/data/releases/pombase-2021-03-01/) using BWA-MEM (version 0.7.17) (Li 2013). The Genome Analysis Toolkit (GATK, version 4.2.0.0) was used for converting SAM files to BAM files, removing duplicate reads, and variant calling (McKenna et al. 2010). The read mapping results were visualized using JBrowse 2 (Hershberg et al. 2021). Hard-filtering thresholds recommended by the GATK team were used to filter false variants. SnpEff (version 5.0) was used for annotating variants (Cingolani et al. 2012). The sequencing data have been deposited under the Project ID CNP0002483 at the sequence archive of China National GeneBank (CNGB) (https://db.cngb.org/search/project/CNP0002483).
Transformation of any1 PCR products: Primers 5′-gcactacctcgttcaaccac-3′ and 5′-tgtttcccgcaacgttcttt-3′ were used to perform PCR amplification from genomic DNAs of a wild-type strain and a can1-1 strain. The PCR products are 934-bp long and encompass most of the coding sequence of the any1 gene. The mutated nucleotide in the any1-R175C PCR product amplified from the can1-1 strain is 507 bp away from one end of the PCR product and 426 bp away from the other end. A wild-type strain, LD331, was transformed separately with equal amounts of the two PCR products. For each transformation, 0.2 OD600 units of transformed cells were plated on an EMM plate containing 100 μg/ml of canavanine and incubated at 30 °C for 7 days. Images of plates were taken by scanning using an Epson Perfection V800 Photo scanner.
Phylogenetic analysis: The sequences of G3DSA:1.20.1740.10 domain-containing proteins were retrieved using Ensembl BioMarts (Kinsella et al. 2011). Sequences were aligned using the online MAFFT server (https://mafft.cbrc.jp/alignment/server/) (Katoh et al. 2019). The E-INS-i iterative refinement algorithm of MAFFT was used. A maximum likelihood tree was calculated using IQ-TREE version 2.1.3 for Mac OS X (Minh et al. 2020). The tree was rooted by midpoint rooting and visualized using FigTree version 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/).
Protein complex structure prediction: The structures of Any1-Cat1 complexes were predicted using AlphaFold-Multimer with default parameters (Evans et al. 2021). Full-length protein sequences were used for prediction. The predicted structures were visualized using PyMOL (version 2.5.0). Among the 5 structures predicted using the 5 different trained models of AlphaFold-Multimer, we chose the one with the highest confidence score. The interface area and the free energy of dissociation of each complex composed of residues 17-338 of Any1 and residues 40-52 of Cat1 were calculated using the PDBePisa web server (https://www.ebi.ac.uk/pdbe/pisa/) (Krissinel and Henrick 2007).
Reagents
| Strain | Genotype | Original strain name | Original source | NBRP ID |
| DY7144 | h− can1-1 | Kohli 10-389 | Jürg Kohli | FY18665 |
| DY8198 | h+ ade7-50 his3-D1 can1-1 leu1-32 | KS3402 | Ken Sawin lab (Anders et al. 2008) | – |
| DY8199 | h− ade7-50 his3-D1 can1-1 leu1-32 | KS3404 | Ken Sawin lab (Anders et al. 2008) | – |
| DY49190 | h− leu1-32 ura4-D18 | PR109 | Paul Russell lab | – |
| DY49191 | h+ leu1-32 | KS1599 | Paul Russell lab | – |
| LD331 | h+ | – | Li-Lin Du | – |
Acknowledgments
We thank the Yeast Genetic Resource Center of Japan (YGRC/NBRP) and Ken Sawin for providing strains.
References
Funding
This work was supported by grants from the Ministry of Science and Technology of the People’s Republic of China and the Beijing municipal government.
Reviewed By
AnonymousHistory
Received: January 25, 2022Revision received: March 5, 2022
Accepted: March 9, 2022
Published: March 14, 2022
Copyright
© 2022 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Yang, YS; Ning, SK; Lyu, XH; Suo, F; Jia, GS; Li, W; Du, LL (2022). Canavanine resistance mutation can1-1 in Schizosaccharomyces pombe is a missense mutation in the ubiquitin ligase adaptor gene any1. microPublication Biology. 10.17912/micropub.biology.000538.Download: RIS BibTeX